The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport
- PMID: 17971878
- PMCID: PMC2040519
- DOI: 10.1371/journal.pone.0001118
The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport
Abstract
Background: Annexin A2 is a peripheral membrane protein that belongs to the annexin family of Ca(2+) and phospholipid-binding proteins. This protein, which plays a role in membrane organization and dynamics in particular along the endocytic pathway, exists as a heterotetrameric complex, consisting of two annexin A2 molecules bound via their N-termini to a dimer of p11/S100A10 light chains. The light chain, and thus presumably formation of the heterotetramer, was reported to control annexin A2 association to the plasma membrane and to cortical actin, as well as the distribution of recycling endosomes. However, the specific role of the light chain and the functions of monomeric versus heterotetrameric annexin A2 have remained elusive in the endocytic pathway.
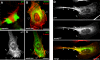
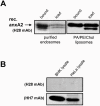
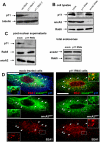
Methodology/principal findings: Here, we have investigated whether p11 plays a role in the endosomal functions of annexin A2. Using morphological and biochemical approaches, we found that p11, unlike annexin A2, was not present on early endosomes. Neither was the heterotetramer detected on purified early endosomes, while it was clearly present in total cell lysates. Moreover, knockdown of p11 with siRNAs did not affect annexin A2 targeting to early endosomes, and, conversely, binding of annexin A2 to purified endosomes or liposomes occurred without p11 in vitro. Finally, while we could confirm that annexin A2 knockdown inhibits transport beyond early endosomes, p11 knockdown had no such effects on early-to-late endosome transport.
Conclusions/significance: Our data show that the binding of annexin A2 to endosomal membranes and its role in endosomal trafficking are independent of the p11/S100A10 light chain. We thus conclude that annexin A2 functions are fully supported by the monomeric form of the protein, at least the endocytic pathway leading to lysosomes.
Conflict of interest statement
Figures

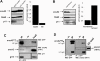
Similar articles
-
Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation.J Biol Chem. 2009 Jan 16;284(3):1604-11. doi: 10.1074/jbc.M806499200. Epub 2008 Nov 5. J Biol Chem. 2009. PMID: 18990701
-
The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes.Mol Biol Cell. 2003 Dec;14(12):4896-908. doi: 10.1091/mbc.e03-06-0387. Epub 2003 Sep 17. Mol Biol Cell. 2003. PMID: 13679511 Free PMC article.
-
S100A10/p11: family, friends and functions.Pflugers Arch. 2008 Jan;455(4):575-82. doi: 10.1007/s00424-007-0313-4. Epub 2007 Jul 19. Pflugers Arch. 2008. PMID: 17638009 Review.
-
Ca2+ ions facilitate the organization of the Annexin A2/S100A10 heterotetramer.Proteins. 2023 Aug;91(8):1042-1053. doi: 10.1002/prot.26490. Epub 2023 Mar 25. Proteins. 2023. PMID: 36965169 Free PMC article.
-
Modulation of Ion Channels and Receptors by p11 (S100A10).Trends Pharmacol Sci. 2020 Jul;41(7):487-497. doi: 10.1016/j.tips.2020.04.004. Epub 2020 May 14. Trends Pharmacol Sci. 2020. PMID: 32418644 Review.
Cited by
-
Moesin and cortactin control actin-dependent multivesicular endosome biogenesis.Mol Biol Cell. 2016 Nov 1;27(21):3305-3316. doi: 10.1091/mbc.E15-12-0853. Epub 2016 Sep 7. Mol Biol Cell. 2016. PMID: 27605702 Free PMC article.
-
The Annexin A2/S100A10 Complex: The Mutualistic Symbiosis of Two Distinct Proteins.Biomolecules. 2021 Dec 9;11(12):1849. doi: 10.3390/biom11121849. Biomolecules. 2021. PMID: 34944495 Free PMC article. Review.
-
BLOC-1 Brings Together the Actin and Microtubule Cytoskeletons to Generate Recycling Endosomes.Curr Biol. 2016 Jan 11;26(1):1-13. doi: 10.1016/j.cub.2015.11.020. Epub 2015 Dec 24. Curr Biol. 2016. PMID: 26725201 Free PMC article.
-
Annexin 2 is not required for human immunodeficiency virus type 1 particle production but plays a cell type-dependent role in regulating infectivity.J Virol. 2010 Oct;84(19):9783-92. doi: 10.1128/JVI.01584-09. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631122 Free PMC article.
-
In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101.Mol Biol Cell. 2008 Nov;19(11):4942-55. doi: 10.1091/mbc.e08-03-0239. Epub 2008 Sep 3. Mol Biol Cell. 2008. PMID: 18768755 Free PMC article.
References
-
- Futter CE, White IJ. Annexins and Endocytosis. Traffic 2007 - PubMed
-
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. - PubMed
-
- Rescher U, Gerke V. Annexins–unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. - PubMed
-
- Glenney JR., Jr Phosphorylation of p36 in vitro with pp60src. Regulation by Ca2+ and phospholipid. FEBS Lett. 1985;192:79–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

