The MDM-2 antagonist nutlin-3 promotes the maturation of acute myeloid leukemic blasts
- PMID: 17971905
- PMCID: PMC2040212
- DOI: 10.1593/neo.07523
The MDM-2 antagonist nutlin-3 promotes the maturation of acute myeloid leukemic blasts
Abstract
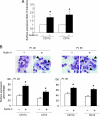
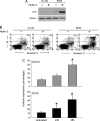
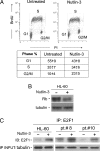
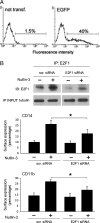
The small-molecule inhibitor of murine double minute (MDM-2), Nutlin-3, induced variable apoptosis in primary acute myeloid leukemia (AML) blasts and promoted myeloid maturation of surviving cells, as demonstrated by analysis of CD11b and CD14 surface antigens and by morphologic examination. Although the best-characterized activity of Nutlin-3 is activation of the p53 pathway, Nutlin-3 induced maturation also in one AML sample characterized by p53 deletion, as well as in the p53(-/-) human myeloblastic HL-60 cell line. At the molecular level, the maturational activity of Nutlin-3 in HL-60 cells was accompanied by the induction of E2F1 transcription factor, and it was significantly counteracted by specific gene knockdown with small interfering RNA for E2F1. Moreover, Nutlin-3, as well as tumor necrosis factor (TNF) alpha, potentiated the maturational activity of recombinant TNF-related apoptosis-inducing ligand (TRAIL) in HL-60 cells. However, although TNF-alpha significantly counteracted the proapoptotic activity of TRAIL, Nutlin-3 did not interfere with the proapoptotic activity of TRAIL. Taken together, these data disclose a novel, potentially relevant therapeutic role for Nutlin-3 in the treatment of both p53 wild-type and p53(-/-) AML, possibly in association with recombinant TRAIL.
Keywords: Acute myeloid leukemia; HL-60 cells; apoptosis; p53 pathway; surface antigens.
Figures


References
-
- Bueso-Ramos CE, Manshouri T, Haidar MA, Huh YO, Keating MJ, Albitar M. Multiple patterns of MDM-2 deregulation in human leukemias: implications in leukemogenesis and prognosis. Leuk Lymphoma. 1995;17:13–18. - PubMed
-
- Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001–1008. - PubMed
-
- Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3–8. - PubMed
-
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
