Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct
- PMID: 17972320
- PMCID: PMC2663799
- DOI: 10.1002/jnr.21561
Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct
Abstract
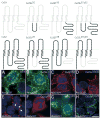
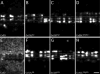
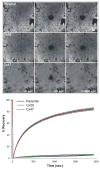
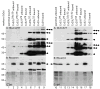
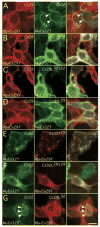
In rodents, oligodendrocytes and myelinating Schwann cells express connexin32 (Cx32) and Cx29, which have different localizations in the two cell types. We show here that, in contrast to Cx32, Cx29 does not form gap junction plaques or functional gap junctions in transfected cells. Furthermore, when expressed together, Cx29 and Cx32 are not colocalized and do not coimmunoprecipitate. To determine the structural basis of their divergent behavior, we generated a series of chimeric Cx32-Cx29 proteins by exchanging their intracellular loops and/or their C-terminal cytoplasmic tails. Although some chimerae reach the cell membrane, others appear to be largely localized intracellularly; none form gap junction plaques or functional gap junctions. Substituting the C-terminus or the intracellular loop and the C-terminus of Cx32 with those of Cx29 does not disrupt their colocalization or coimmunoprecipitation with Cx32. Substituting the C-terminus of Cx29 with that of Cx32 does not disrupt the coimmunoprecipitation or the colocalization with Cx29, whereas substituting both the intracellular loop and the C-terminus of Cx32 with those of Cx29 diminishes the coimmunoprecipitation with Cx29. Conversely, the Cx32 chimera that contains the intracellular loop of Cx29 coimmunoprecipitates with Cx29, indicating that the intracellular loop participates in Cx29-Cx29 interactions. These data indicate that homomeric interactions of Cx29 and especially Cx32 largely require other domains: the N-terminus, transmembrane domains, and extracellular loops. Substituting the intracellular loop and/or tail of Cx32 with those of Cx29 appears to prevent Cx32 from forming functional gap junctions.
Figures


References
-
- Abrams CK, Oh S, Ri Y, Bargiello TA. Mutations in connexin 32: the molecular and biophysical bases for the X-linked form of Charcot- Marie-Tooth disease. Brain Res Rev. 2000;32:203–214. - PubMed
-
- Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, DAndrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun. 2003;305:1024–1033. - PubMed
-
- Bone Jeng LJ, Messing A, Balice-Gordon R, Fischbeck KH, Scherer SS. The effects of a dominant connexin32 mutant in myelinating Schwann cells. Mol Cell Neurosci. 2006;32:283–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

