Get to grips: steering local actin dynamics with IQGAPs
- PMID: 17972901
- PMCID: PMC2247391
- DOI: 10.1038/sj.embor.7401089
Get to grips: steering local actin dynamics with IQGAPs
Abstract
IQGAPs are actin-binding proteins that scaffold numerous interaction partners, transmitting extracellular signals that influence mitogenic, morphological and migratory cell behaviour. However, the precise mechanisms by which IQGAP proteins influence actin dynamics and actin filament structures have been elusive. Now that IQGAP1 has emerged as a potential key regulator of actin-cytoskeletal dynamics by recruiting both the actin related protein (Arp)2/3 complex and/or formin-dependent actin polymerizing machineries, we propose that IQGAP1 might coordinate the function of mechanistically different actin nucleators for cooperative localized actin filament production in various cellular processes.
Figures
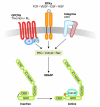
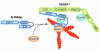
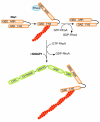


References
-
- Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, Bloom GS (2007) IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci 120: 658–669 - PubMed
-
- Brown MD, Sacks DB (2006) IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol 16: 242–249 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

