A role for cytochrome c and cytochrome c peroxidase in electron shuttling from Erv1
- PMID: 17972915
- PMCID: PMC2099471
- DOI: 10.1038/sj.emboj.7601909
A role for cytochrome c and cytochrome c peroxidase in electron shuttling from Erv1
Abstract
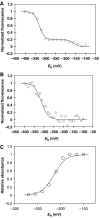
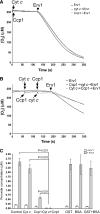
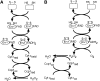
Erv1 is a flavin-dependent sulfhydryl oxidase in the mitochondrial intermembrane space (IMS) that functions in the import of cysteine-rich proteins. Redox titrations of recombinant Erv1 showed that it contains three distinct couples with midpoint potentials of -320, -215, and -150 mV. Like all redox-active enzymes, Erv1 requires one or more electron acceptors. We have generated strains with erv1 conditional alleles and employed biochemical and genetic strategies to facilitate identifying redox pathways involving Erv1. Here, we report that Erv1 forms a 1:1 complex with cytochrome c and a reduced Erv1 can transfer electrons directly to the ferric form of the cytochrome. Erv1 also utilized molecular oxygen as an electron acceptor to generate hydrogen peroxide, which is subsequently reduced to water by cytochrome c peroxidase (Ccp1). Oxidized Ccp1 was in turn reduced by the Erv1-reduced cytochrome c. By coupling these pathways, cytochrome c and Ccp1 function efficiently as Erv1-dependent electron acceptors. Thus, we propose that Erv1 utilizes diverse pathways for electron shuttling in the IMS.
Figures





References
-
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K (2005) Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol 353: 937–944 - PubMed
-
- Allen S, Lu H, Thornton D, Tokatlidis K (2003) Juxtaposition of the two distal ‘CX3C' motifs via intrachain disulfide bonding is essential for the folding of Tim10. J Biol Chem 278: 38505–38513 - PubMed
-
- Bader M, Muse W, Ballou DP, Gassner C, Bardwell JC (1999) Oxidative protein folding is driven by the electron transport system. Cell 98: 217–227 - PubMed
-
- Bosshard HR, Davidson MW, Knaff DB, Millett F (1986) Complex formation and electron transfer between mitochondrial cytochrome c and flavocytochrome c552 from Chromatium vinosum. J Biol Chem 261: 190–193 - PubMed
-
- Boveris A (1976) Mitochondrial production of hydrogen peroxide in Saccharomyces cerevisiae. Acta Physiol Lat Am 26: 303–309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

