Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study
- PMID: 17974004
- PMCID: PMC2186303
- DOI: 10.1186/1746-1596-2-42
Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study
Abstract
Context: Myeloid sarcoma (MS) is a neoplasm of immature granulocytes, monocytes, or both involving any extramedullary site. The correct diagnosis of MS is important for adequate therapy, which is often delayed because of a high misdiagnosis rate.
Objective: To evaluate the lineage differentiation of neoplastic cells in MS by immunohistochemistry, and to correlate the results with clinicopathologic findings and cytogenetic studies.
Design: Histologic and immunohistochemical examinations were performed on formalin-fixed paraffin-embedded tissue samples from 13 cases of MS. They were classified according to the World Health Organization criteria. Chromosomal analysis data were available in 11 cases. Clinical, pathological, and cytogenetic findings were analyzed.
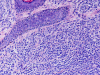
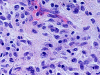
Results: The study included six male and seven female patients with an age range of 25 to 72 years (mean, 49.3 years) and a male to female ratio of 1:1.2. MS de novo occurred in 4/13 (31%) of cases examined. The most sensitive immunohistochemical markers were CD43 and lysozyme present in all cases with MS (13/13, 100%). All de novo MS showed a normal karyotype, monoblastic differentiation, and lack of CD34. The most common chromosomal abnormalities in MS associated with a hematopoietic disorder were trisomy 8 and inv(16) (2/11, 18%).
Conclusion: An immunohistochemical panel including CD43, lysozyme, myeloperoxidase (MPO), CD68 (or CD163), CD117, CD3 and CD20 can successfully identify the vast majority of MS variants in formalin-fixed paraffin-embedded tissue sections. The present report expands the spectrum of our knowledge showing that de novo MS has frequent monoblastic differentiation and frequently carries a normal karyotype.
Figures









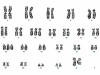
References
-
- Brunnung RD, Matutes E, Flandrin G, Vardiman J, Bennett J, Head D, Harris NL. Acute myeloid leukemias. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editor. World Health Organization Classification of Tumors Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissue. IARC Press; 2001. pp. 77–105.
-
- Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, Thomas DA, Garcia-Manero G, Ferrajoli A, Manning JT, Keating MJ, Albitar M, O'Brien S, Giles FJ. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003;17:1100–1103. doi: 10.1038/sj.leu.2402958. - DOI - PubMed
-
- Zekry N, Klooster MJ, Raghavan R, Wang J. A 7-year-old child with a history of acute myeloid leukemia presenting with multiple gastrointestinal polyps. Arch Pathol Lab Med. 2006;130:3–4. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

