Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse
- PMID: 17974006
- PMCID: PMC2186315
- DOI: 10.1186/1743-422X-4-118
Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse
Abstract
Background: The M2 ectodomain (M2e) of influenza A virus (IAV) strains that have circulated in humans during the past 90 years shows remarkably little structural diversity. Since M2e-specific antibodies (Abs) are capable of restricting IAV replication in vivo but are present only at minimal concentration in human sera, efforts are being made to develop a M2e-specific vaccine. We are exploring a synthetic multiple antigenic peptide (MAP) vaccine and here report on the role of adjuvants (cholera toxin and immunostimulatory oligodeoxynucleotide) and route of immunization on Ab response and strength of protection.
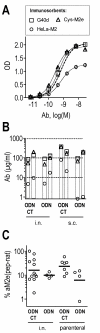
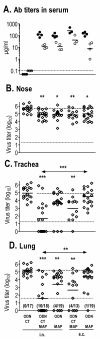
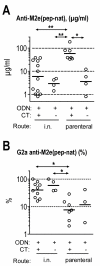
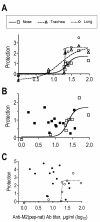
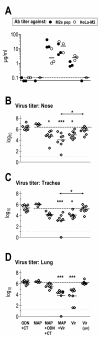
Results: Independent of adjuvants and immunization route, on average 87% of the M2e-MAP-induced Abs were specific for M2e peptide and a variable fraction of these M2e(pep)-specific Abs (average 15%) cross-reacted with presumably native M2e expressed by M2-transfected cells. The titer of these cross-reactive M2e(pep-nat)-specific Abs in sera of parenterally immunized mice displayed a sigmoidal relation to level of protection, with EC50 of approximately 20 microg Ab/ml serum, though experiments with passive M2e(pep-nat) Abs indicated that serum Abs did not fully account for protection in parenterally vaccinated mice, particularly in upper airways. Intranasal vaccination engendered stronger protection and a higher proportion of G2a Abs than parenteral vaccination, and the strength of protection failed to correlate with M2e(pep-nat)-specific serum Ab titers, suggesting a role of airway-associated immunity in protection of intranasally vaccinated mice. Intranasal administration of M2e-MAP without adjuvant engendered no response but coadministration with infectious IAV slightly enhanced the M2e(pep-nat) Ab response and protection compared to vaccination with IAV or adjuvanted M2e-MAP alone.
Conclusion: M2e-MAP is an effective immunogen as approximately 15% of the total M2e-MAP-induced Ab response is of desired specificity. While M2e(pep-nat)-specific serum Abs have an important role in restricting virus replication in trachea and lung, M2e-specific T cells and/or locally produced Abs contribute to protection in upper airways. Intranasal vaccination is preferable to parenteral vaccination, presumably because of induction of local protective immunity by the former route. Intranasal coadministration of M2e-MAP with infectious IAV merits further investigation in view of its potential applicability to human vaccination with live attenuated IAV.
Figures


Similar articles
-
Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice.J Virol. 2014 Sep 1;88(17):9693-703. doi: 10.1128/JVI.00823-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920793 Free PMC article.
-
Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza.PLoS One. 2011;6(11):e27953. doi: 10.1371/journal.pone.0027953. Epub 2011 Nov 30. PLoS One. 2011. PMID: 22140491 Free PMC article.
-
Heterosubtypic protection conferred by combined vaccination with M2e peptide and split influenza vaccine.Vaccine. 2009 Oct 9;27(43):6095-101. doi: 10.1016/j.vaccine.2008.11.037. Epub 2008 Dec 3. Vaccine. 2009. PMID: 19056447
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Influenza A viruses: why focusing on M2e-based universal vaccines.Virus Genes. 2011 Feb;42(1):1-8. doi: 10.1007/s11262-010-0547-7. Epub 2010 Nov 17. Virus Genes. 2011. PMID: 21082230 Review.
Cited by
-
Vaccines: the fourth century.Clin Vaccine Immunol. 2009 Dec;16(12):1709-19. doi: 10.1128/CVI.00290-09. Epub 2009 Sep 30. Clin Vaccine Immunol. 2009. PMID: 19793898 Free PMC article. Review.
-
Intranasal Vaccination With Lipoproteins Confers Protection Against Pneumococcal Colonisation.Front Immunol. 2018 Oct 18;9:2405. doi: 10.3389/fimmu.2018.02405. eCollection 2018. Front Immunol. 2018. PMID: 30405609 Free PMC article.
-
Mucosal vaccination with recombinant Lactobacillus casei-displayed CTA1-conjugated consensus matrix protein-2 (sM2) induces broad protection against divergent influenza subtypes in BALB/c mice.PLoS One. 2014 Apr 8;9(4):e94051. doi: 10.1371/journal.pone.0094051. eCollection 2014. PLoS One. 2014. PMID: 24714362 Free PMC article.
-
Immunological assessment of influenza vaccines and immune correlates of protection.Expert Rev Vaccines. 2013 May;12(5):519-36. doi: 10.1586/erv.13.35. Expert Rev Vaccines. 2013. PMID: 23659300 Free PMC article. Review.
-
Influenza virus-like particles containing M2 induce broadly cross protective immunity.PLoS One. 2011 Jan 18;6(1):e14538. doi: 10.1371/journal.pone.0014538. PLoS One. 2011. PMID: 21267073 Free PMC article.
References
-
- Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox N, Strikas RA, Centers for Disease Control and Prevention (U.S.), Practices USACoI . Prevention and control of influenza : recommendations of the Advisory Committee on Immunization Practices (ACIP) Atlanta, GA: U.S. Dept. of Health & Human Services Centers for Disease Control and Prevention; 2006. - PubMed
-
- Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis. 1980;142:844–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

