Comparison of glucosamine sulfate and a polyherbal supplement for the relief of osteoarthritis of the knee: a randomized controlled trial [ISRCTN25438351]
- PMID: 17974032
- PMCID: PMC2131759
- DOI: 10.1186/1472-6882-7-34
Comparison of glucosamine sulfate and a polyherbal supplement for the relief of osteoarthritis of the knee: a randomized controlled trial [ISRCTN25438351]
Abstract
Background: The efficacy and safety of a dietary supplement derived from South American botanicals was compared to glucosamine sulfate in osteoarthritis subjects in a Mumbai-based multi-center, randomized, double-blind study.
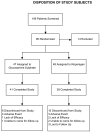
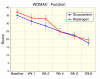
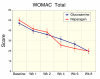
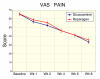
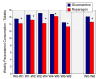
Methods: Subjects (n = 95) were screened and randomized to receive glucosamine sulfate (n = 47, 1500 mg/day) or reparagen (n = 48, 1800 mg/day), a polyherbal consisting of 300 mg of vincaria (Uncaria guianensis) and 1500 mg of RNI 249 (Lepidium meyenii) administered orally, twice daily. Primary efficacy variable was response rate based on a 20% improvement in WOMAC pain scores. Additional outcomes were WOMAC scores for pain, stiffness and function, visual analog score (VAS) for pain, with assessments at 1, 2, 4, 6 and 8 weeks. Tolerability, investigator and subject global assessments and rescue medication consumption (paracetamol) were measured together with safety assessments including vital signs and laboratory based assays.
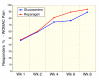
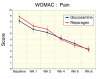
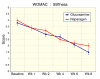
Results: Subject randomization was effective: age, gender and disease status distribution was similar in both groups. The response rates (20% reduction in WOMAC pain) were substantial for both glucosamine (89%) and reparagen (94%) and supported by investigator and subject assessments. Using related criteria response rates to reparagen were favorable when compared to glucosamine. Compared to baseline both treatments showed significant benefits in WOMAC and VAS outcomes within one week (P < 0.05), with a similar, progressive improvement over the course of the 8 week treatment protocol (45-62% reduction in WOMAC or VAS scores). Tolerability was excellent, no serious adverse events were noted and safety parameters were unchanged. Rescue medication use was significantly lower in the reparagen group (p < 0.01) at each assessment period. Serum IGF-1 levels were unaltered by treatments.
Conclusion: Both reparagen and glucosamine sulfate produced substantial improvements in pain, stiffness and function in subjects with osteoarthritis. Response rates were high and the safety profile was excellent, with significantly less rescue medication use with reparagen. Reparagen represents a new natural productive alternative in the management of joint health.
Trial registration: Current Controlled Trials ISRCTN25438351.
Figures
References
-
- Murray CJL, Lopez AD. The Global Burden of Disease. Boston: Harvard University Press; 1996.
-
- Grainger R, Cicuttini FM. Medical management of osteoarthritis of the knee and hip joints. Med J Aust. 2004;180:232–236. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

