A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy
- PMID: 17974583
- PMCID: PMC2375552
- DOI: 10.1113/jphysiol.2007.141507
A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy
Abstract
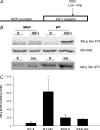
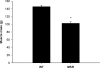
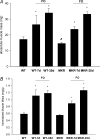
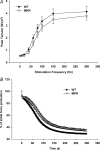
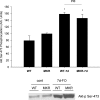
Increasing the mechanical load on skeletal muscle results in increased expression of insulin-like growth factor I (IGF-I), which is thought to be a critical step in the induction of muscle hypertrophy. To determine the role of the IGF-I receptor in load-induced skeletal muscle hypertrophy, we utilized a transgenic mouse model (MKR) that expresses a dominant negative IGF-I receptor specifically in skeletal muscle. Skeletal muscle hypertrophy was induced in the plantaris muscle using the functional overload (FO) model, a model which has previously been shown to induce significant elevations of IGF-I expression in skeletal muscle. Adult male wild-type (WT) and MKR mice were subjected to 0, 7 or 35 days of FO. In control or unchallenged animals, the plantaris mass was 11% greater in WT compared to the MKR mice (P < 0.05). After 7 days of FO, plantaris mass increased significantly by 26% and 62% in WT and MKR mice, respectively (P < 0.05). After 35 days of FO, WT and MKR mice demonstrated significant increases of 100% and 122%, respectively, in plantaris mass (P < 0.05). Further, at no time point was the degree of hypertrophy significantly different between the WT and MKR mice. Previous research suggests that IGF-I induces muscle growth through activation of the Akt-mTOR signalling pathway; therefore, we measured the phosphorylation status of Akt and p70(s6k) in the WT and MKR mice after 7 days of FO. Significant increases of approximately 100% and approximately 200% in Akt (Ser-473) and p70(s6k) (Thr-389) phosphorylation were measured in overloaded plantaris from both WT and MKR mice, respectively. Moreover, no differences were detected between the WT and MKR mice. These data suggest that increased mechanical load can induce muscle hypertrophy and activate the Akt and p70(s6k) independent of a functioning IGF-I receptor.
Figures

Comment in
-
Muscle growth: no IGFs, ands, or buts.J Physiol. 2008 Jan 1;586(1):5-6. doi: 10.1113/jphysiol.2007.147660. J Physiol. 2008. PMID: 18167368 Free PMC article. No abstract available.
References
-
- Adams GR. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. - PubMed
-
- Adams GR, Haddad F. The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996;81:2509–2516. - PubMed
-
- Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722. - PubMed
-
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

