Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils
- PMID: 17974994
- PMCID: PMC2919236
- DOI: 10.1158/0008-5472.CAN-07-1778
Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils
Abstract
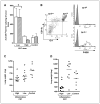
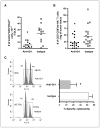
Photodynamic therapy (PDT) is a Food and Drug Administration-approved local cancer treatment that can be curative of early disease and palliative in advanced disease. PDT of murine tumors results in regimen-dependent induction of an acute local inflammatory reaction, characterized in part by rapid neutrophil infiltration into the treated tumor bed. In this study, we show that a PDT regimen that induced a high level of neutrophilic infiltrate generated tumor-specific primary and memory CD8(+) T-cell responses. In contrast, immune cells isolated from mice treated with a PDT regimen that induced little or no neutrophilic infiltrate exhibited minimal antitumor immunity. Mice defective in neutrophil homing to peripheral tissues (CXCR2(-/-) mice) or mice depleted of neutrophils were unable to mount strong antitumor CD8(+) T-cell responses following PDT. Neutrophils seemed to be directly affecting T-cell proliferation and/or survival rather than dendritic cell maturation or T-cell migration. These novel findings indicate that by augmenting T-cell proliferation and/or survival, tumor-infiltrating neutrophils play an essential role in establishment of antitumor immunity following PDT. Furthermore, our results may suggest a mechanism by which neutrophils might affect antitumor immunity following other inflammation-inducing cancer therapies. Our findings lay the foundation for the rational design of PDT regimens that lead to optimal enhancement of antitumor immunity in a clinical setting. Immune-enhancing PDT regimens may then be combined with treatments that result in optimal ablation of primary tumors, thus inhibiting growth of primary tumor and controlling disseminated disease.
Figures



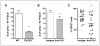
References
-
- Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol. 2007;19:48–55. - PubMed
-
- Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–6. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

