TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis
- PMID: 17975002
- PMCID: PMC10068840
- DOI: 10.1158/0008-5472.CAN-07-1356
TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis
Abstract
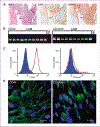
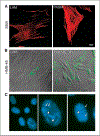
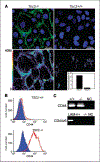
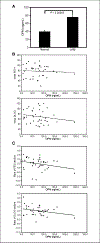
Lymphangioleiomyomatosis (LAM), a rare multisystem disease found primarily in women of childbearing age, is characterized by the proliferation of abnormal smooth muscle-like cells, LAM cells, that form nodules in the pulmonary interstitium. Proliferation of LAM cells results, in part, from dysfunction in tuberous sclerosis complex (TSC) genes TSC1 (hamartin) and/or TSC2 (tuberin). Identification of LAM cells in donor lungs, their isolation from blood, and their presence in urine, chylous ascites, and pleural effusions are consistent with their ability to metastasize. Here, we investigated the presence on LAM cells of the hyaluronic acid receptor CD44 and its splice variants associated with metastasis. The heterogeneous populations of cells grown from lungs of 12 LAM patients contain cells expressing mRNA for the variant CD44v6. Histologically, CD44v6 was present in LAM lung nodules, but not in normal vascular smooth muscle cells. CD44v6-positive sorted cells showed loss of heterozygosity at the TSC2 locus; binding of CD44v6 antibody resulted in loss of cell viability. Levels of CD44 were higher in cultured Eker rat (Tsc2-/-) cells than in Tsc2+/+ cells, but unlike human LAM cells, the Tsc2-/- Eker rat cells did not contain CD44v6 splice variant mRNA. CD44 splicing and signaling is regulated by osteopontin. Plasma from LAM patients contained higher concentrations of osteopontin than plasma of healthy, age-, and sex-matched volunteers (P = 0.00003) and may be a biomarker for LAM. The cell surface receptor CD44 and its splice variant CD44v6 may contribute to the metastatic potential of LAM cells.
Figures

References
-
- Pacheco-Rodriguez G, Kristof AS, Stevens LA, et al. Giles F. Filley Lecture. Genetics and gene expression in lymphangioleiomyomatosis. Chest 2002;121:56–60S. - PubMed
-
- Jóźwiak S, Schwartz RA, Janniger CK, Michalowicz R, Chmielik J. Skin lessions in children with tuberous sclerosis complex: their prevalence, natural course, and diagnostic significance. Int J Derm 1998;37:911–7. - PubMed
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

