Protein aggregation and proteasome dysfunction after brain ischemia
- PMID: 17975104
- PMCID: PMC2670397
- DOI: 10.1161/STROKEAHA.107.487108
Protein aggregation and proteasome dysfunction after brain ischemia
Abstract
Background and purpose: Protein unfolding and aggregation are dominant early pathogenic events in neurons after brain ischemia. This study used a transient cerebral ischemia model to investigate whether overproduction of unfolded proteins after brain ischemia is a consequence of proteasome dysfunction.
Methods: Proteasome peptidase activity and proteasome subcellular redistribution and assembly were studied by peptidase activity assay, Western blot analysis, and size-exclusion chromatography.
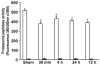
Results: Proteasome peptidase activity, as determined with the peptide substrate succinyl-LLVY-7-amino-4-methylcoumarin, was moderately decreased, and the 26S proteasome was disassembled during the early period of reperfusion after transient brain ischemia. Furthermore, the proteasome subunits, particularly the 19S components, were deposited into the protein aggregate-containing fraction after an episode of transient cerebral ischemia.
Conclusions: These results clearly demonstrate that after an episode of brain ischemia, proteasomes are disassembled and aggregated and thus fail to function normally. Deposition of proteasomes into protein aggregates may also indicate that proteasomes attempt to degrade ubiquitin-conjugated proteins (ubiproteins) overproduced after brain ischemia. However, ubiproteins are too numerous to be degraded and trap some of the proteasomes into their aggregates after brain ischemia.
Figures


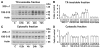

Similar articles
-
Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion.Stroke. 2012 Aug;43(8):2229-35. doi: 10.1161/STROKEAHA.112.650416. Epub 2012 Jun 14. Stroke. 2012. PMID: 22700531 Free PMC article.
-
Role of the ubiquitin-proteasome system in brain ischemia: friend or foe?Prog Neurobiol. 2014 Jan;112:50-69. doi: 10.1016/j.pneurobio.2013.10.003. Epub 2013 Oct 22. Prog Neurobiol. 2014. PMID: 24157661 Review.
-
Ischemic postconditioning protects neuronal death caused by cerebral ischemia and reperfusion via attenuating protein aggregation.Int J Med Sci. 2012;9(10):923-32. doi: 10.7150/ijms.4878. Epub 2012 Nov 23. Int J Med Sci. 2012. PMID: 23236262 Free PMC article.
-
Trehalose Inhibits Protein Aggregation Caused by Transient Ischemic Insults Through Preservation of Proteasome Activity, Not via Induction of Autophagy.Mol Neurobiol. 2017 Nov;54(9):6857-6869. doi: 10.1007/s12035-016-0196-5. Epub 2016 Oct 22. Mol Neurobiol. 2017. PMID: 27771898
-
Protein degradation pathways after brain ischemia.Curr Drug Targets. 2012 Feb;13(2):159-65. doi: 10.2174/138945012799201694. Curr Drug Targets. 2012. PMID: 22204315 Review.
Cited by
-
Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischemic stroke.Cell Death Dis. 2015 Jan 29;6(1):e1626. doi: 10.1038/cddis.2014.586. Cell Death Dis. 2015. PMID: 25633295 Free PMC article.
-
Life and death in the trash heap: The ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral Ischemia.Ageing Res Rev. 2017 Mar;34:30-38. doi: 10.1016/j.arr.2016.09.011. Epub 2016 Oct 1. Ageing Res Rev. 2017. PMID: 27702698 Free PMC article. Review.
-
Subcellular stress response and induction of molecular chaperones and folding proteins after transient global ischemia in rats.Brain Res. 2009 Jan 16;1249:9-18. doi: 10.1016/j.brainres.2008.10.032. Epub 2008 Oct 28. Brain Res. 2009. PMID: 18996359 Free PMC article.
-
Loss of endoplasmic reticulum Ca2+ homeostasis: contribution to neuronal cell death during cerebral ischemia.Acta Pharmacol Sin. 2013 Jan;34(1):49-59. doi: 10.1038/aps.2012.139. Epub 2012 Oct 29. Acta Pharmacol Sin. 2013. PMID: 23103622 Free PMC article. Review.
-
Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion.Stroke. 2012 Aug;43(8):2229-35. doi: 10.1161/STROKEAHA.112.650416. Epub 2012 Jun 14. Stroke. 2012. PMID: 22700531 Free PMC article.
References
-
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. - PubMed
-
- Meredith SC. Protein denaturation and aggregation: cellular responses to denatured and aggregated proteins. Ann N Y Acad Sci. 2005;1066:181–221. - PubMed
-
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. - PubMed
-
- Siesjo BK, Elmer E, Janelidze S, Keep M, Kristian T, Ouyang YB, Uchino H. Role and mechanisms of secondary mitochondrial failure. Acta Neurochir Suppl. 1999;73:7–13. - PubMed
-
- Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjo BK, Liu CL. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab. 2001;21:865–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

