Förster transfer recovery reveals that phospholamban exchanges slowly from pentamers but rapidly from the SERCA regulatory complex
- PMID: 17975108
- PMCID: PMC2590498
- DOI: 10.1161/CIRCRESAHA.107.159947
Förster transfer recovery reveals that phospholamban exchanges slowly from pentamers but rapidly from the SERCA regulatory complex
Abstract
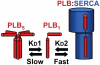
Phospholamban (PLB) or the sarcoplasmic reticulum Ca2+-ATPase (SERCA) were fused to cyan fluorescent protein (CFP) and coexpressed with PLB fused to yellow fluorescent protein (YFP). The expressed fluorescently tagged proteins were imaged using epifluorescence and total internal reflection fluorescence microscopy. YFP fluorescence was selectively bleached by a focused laser beam. CFP fluorescence at the targeted site increased after YFP photobleaching, indicating fluorescence resonance energy transfer between CFP-SERCA/CFP-PLB and YFP-PLB. The increased donor fluorescence relaxed back toward baseline as a result of donor diffusion and exchange of bleached YFP-PLB for unbleached YFP-PLB, which restored fluorescence resonance energy transfer. Requenching of CFP donors, termed Förster transfer recovery (FTR), was quantified as an index of the rate of PLB subunit exchange from the PLB:SERCA and PLB:PLB membrane complexes. PLB subunit exchange from the PLB:SERCA regulatory complex was rapid, showing diffusion-limited FTR (tau=1.4 second). Conversely, PLB:PLB oligomeric complexes were found to be stable on a much longer time scale. Despite free lateral diffusion in the membrane, they showed no FTR over 80 seconds. Mutation of PLB position 40 from isoleucine to alanine (I40A-PLB) did not abolish PLB:PLB energy transfer, but destabilization of the PLB:PLB complex was apparent from an increased FTR rate (tau=8.4 seconds). Oligomers of I40A-PLB were stabilized by oxidative crosslinking of transmembrane cysteines with diamide. We conclude that PLB exchanges rapidly from its regulatory complex with the SERCA pump, whereas subunit exchange from the PLB oligomeric complex is slow and does not occur on the time scale of the cardiac cycle.
Figures






References
-
- MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. - PubMed
-
- Asahi M, McKenna E, Kurzydlowski K, Tada M, MacLennan DH. Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J Biol Chem. 2000;275:15034–15038. - PubMed
-
- Chen Z, Akin BL, Stokes DL, Jones LR. Cross-linking of C-terminal residues of phospholamban to the Ca2+ pump of cardiac sarcoplasmic reticulum to probe spatial and functional interactions within the transmembrane domain. J Biol Chem. 2006;281:14163–14172. - PubMed
-
- Li J, Bigelow DJ, Squier TC. Conformational changes within the cytosolic portion of phospholamban upon release of Ca-ATPase inhibition. Biochemistry. 2004;43:3870–3879. - PubMed
-
- Mueller B, Karim CB, Negrashov IV, Kutchai H, Thomas DD. Direct detection of phospholamban and sarcoplasmic reticulum Ca-ATPase interaction in membranes using fluorescence resonance energy transfer. Biochemistry. 2004;43:8754–8765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

