Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice
- PMID: 17975669
- PMCID: PMC2045593
- DOI: 10.1172/JCI31726
Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice
Abstract
The skin is the first line of defense against microbial infection, and psychological stress (PS) has been shown to have adverse effects on cutaneous barrier function. Here we show that PS increased the severity of group A Streptococcus pyogenes (GAS) cutaneous skin infection in mice; this was accompanied by increased production of endogenous glucocorticoids (GCs), which inhibited epidermal lipid synthesis and decreased lamellar body (LB) secretion. LBs encapsulate antimicrobial peptides (AMPs), and PS or systemic or topical GC administration downregulated epidermal expression of murine AMPs cathelin-related AMP and beta-defensin 3. Pharmacological blockade of the stress hormone corticotrophin-releasing factor or of peripheral GC action, as well as topical administration of physiologic lipids, normalized epidermal AMP levels and delivery to LBs and decreased the severity of GAS infection during PS. Our results show that PS decreases the levels of 2 key AMPs in the epidermis and their delivery into LBs and that this is attributable to increased endogenous GC production. These data suggest that GC blockade and/or topical lipid administration could normalize cutaneous antimicrobial defense during PS or GC increase. We believe this to be the first mechanistic link between PS and increased susceptibility to infection by microbial pathogens.
Figures

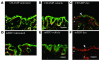

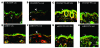
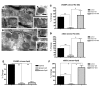

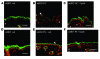


Comment in
-
A nervous breakdown in the skin: stress and the epidermal barrier.J Clin Invest. 2007 Nov;117(11):3166-9. doi: 10.1172/JCI33508. J Clin Invest. 2007. PMID: 17975659 Free PMC article.
References
-
- Glaser R. Stress-associated immune dysregulation and its importance for human health: a personal history of psychoneuroimmunology. Brain Behav. Immun. 2005;19:3–11. - PubMed
-
- Meddings J.B., Swain M.G. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. - PubMed
-
- Jones M.P. The role of psychosocial factors in peptic ulcer disease: beyond Helicobacter pylori and NSAIDs. J. Psychosom. Res. 2006;60:407–412. - PubMed
-
- Strike P.C., Steptoe A. Psychosocial factors in the development of coronary artery disease. Prog. Cardiovasc. Dis. 2004;46:337–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

