Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax
- PMID: 17977963
- PMCID: PMC2224576
- DOI: 10.1128/JVI.01706-07
Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax
Abstract
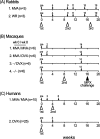
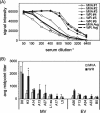
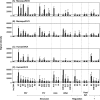
Modified vaccinia virus Ankara (MVA) is a highly attenuated vaccinia virus that is under consideration as an alternative to the conventional smallpox vaccine Dryvax. MVA was attenuated by extensive passage of vaccinia virus Ankara in chicken embryo fibroblasts. Several immunomodulatory genes and genes that influence host range are deleted or mutated, and replication is aborted in the late stage of infection in most nonavian cells. The effect of these mutations on immunogenicity is not well understood. Since the structural genes appear to be intact in MVA, it is hypothesized that critical targets for antibody neutralization have been retained. To test this, we probed microarrays of the Western Reserve (WR) proteome with sera from humans and macaques after MVA and Dryvax vaccination. As most protein sequences of MVA are 97 to 99% identical to those of other vaccinia virus strains, extensive binding cross-reactivity is expected, except for those deleted or truncated. Despite different hosts and immunization regimens, the MVA and Dryvax antibody profiles were broadly similar, with antibodies against membrane and core proteins being the best conserved. The responses to nonstructural proteins were less well conserved, although these are not expected to influence virus neutralization. The broadest antibody response was obtained for hyperimmune rabbits with WR, which is pathogenic in rabbits. These data indicate that, despite the mutations and deletions in MVA, its overall immunogenicity is broadly comparable to that of Dryvax, particularly at the level of antibodies to membrane proteins. The work supports other information suggesting that MVA may be a useful alternative to Dryvax.
Figures






References
-
- Abdalrhman, I., I. Gurt, and E. Katz. 2006. Protection induced in mice against a lethal orthopox virus by the Lister strain of vaccinia virus and modified vaccinia virus Ankara (MVA). Vaccine 244152-4160. - PubMed
-
- Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211320-337. - PubMed
-
- Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244365-396. - PubMed
-
- Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 1009458-9463. - PMC - PubMed
-
- Carroll, M. W., and B. Moss. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238198-211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

