Varicella-zoster virus glycoprotein M homolog is glycosylated, is expressed on the viral envelope, and functions in virus cell-to-cell spread
- PMID: 17977964
- PMCID: PMC2224567
- DOI: 10.1128/JVI.01722-07
Varicella-zoster virus glycoprotein M homolog is glycosylated, is expressed on the viral envelope, and functions in virus cell-to-cell spread
Abstract
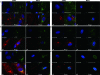
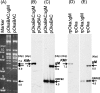
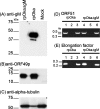
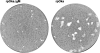
Although envelope glycoprotein M (gM) is highly conserved among herpesviruses, the varicella-zoster virus (VZV) gM homolog has never been investigated. Here we characterized the VZV gM homolog and analyzed its function in VZV-infected cells. The VZV gM homolog was expressed on virions as a glycoprotein modified with a complex N-linked oligosaccharide and localized mainly to the Golgi apparatus and the trans-Golgi network in infected cells. To analyze its function, a gM deletion mutant was generated using the bacterial artificial chromosome system in Escherichia coli, and the virus was reconstituted in MRC-5 cells. VZV is highly cell associated, and infection proceeds mostly by cell-to-cell spread. Compared with wild-type VZV, the gM deletion mutant showed a 90% reduction in plaque size and 50% of the cell-to-cell spread in MRC-5 cells. The analysis of infected cells by electron microscopy revealed numerous aberrant vacuoles containing electron-dense materials in cells infected with the deletion mutant virus but not in those infected with wild-type virus. However, enveloped immature particles termed L particles were found at the same level on the surfaces of cells infected with either type of virus, indicating that envelopment without a capsid might not be impaired. These results showed that VZV gM is important for efficient cell-to-cell virus spread in cell culture, although it is not essential for virus growth.
Figures





Similar articles
-
Varicella-zoster virus glycoprotein M.Curr Top Microbiol Immunol. 2010;342:147-54. doi: 10.1007/82_2010_30. Curr Top Microbiol Immunol. 2010. PMID: 20373090 Review.
-
ORF7 of Varicella-Zoster Virus Is Required for Viral Cytoplasmic Envelopment in Differentiated Neuronal Cells.J Virol. 2017 May 26;91(12):e00127-17. doi: 10.1128/JVI.00127-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28356523 Free PMC article.
-
Characterization of the varicella-zoster virus ORF50 gene, which encodes glycoprotein M.J Virol. 2010 Apr;84(7):3488-502. doi: 10.1128/JVI.01838-09. Epub 2010 Jan 27. J Virol. 2010. PMID: 20106918 Free PMC article.
-
Role of the varicella-zoster virus gB cytoplasmic domain in gB transport and viral egress.J Virol. 2002 Jan;76(2):591-9. doi: 10.1128/jvi.76.2.591-599.2002. J Virol. 2002. PMID: 11752150 Free PMC article.
-
Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus.Rev Med Virol. 2003 Jul-Aug;13(4):207-22. doi: 10.1002/rmv.377. Rev Med Virol. 2003. PMID: 12820183 Review.
Cited by
-
Red-mediated transposition and final release of the mini-F vector of a cloned infectious herpesvirus genome.PLoS One. 2009 Dec 4;4(12):e8178. doi: 10.1371/journal.pone.0008178. PLoS One. 2009. PMID: 19997639 Free PMC article.
-
Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor.PLoS Pathog. 2010 Jul 1;6(7):e1000971. doi: 10.1371/journal.ppat.1000971. PLoS Pathog. 2010. PMID: 20617166 Free PMC article.
-
The varicella-zoster virus genome.Curr Top Microbiol Immunol. 2010;342:1-14. doi: 10.1007/82_2010_10. Curr Top Microbiol Immunol. 2010. PMID: 20225013 Free PMC article. Review.
-
Glycosylation of viral proteins: Implication in virus-host interaction and virulence.Virulence. 2022 Dec;13(1):670-683. doi: 10.1080/21505594.2022.2060464. Virulence. 2022. PMID: 35436420 Free PMC article. Review.
-
Varicella-zoster virus ORF49 functions in the efficient production of progeny virus through its interaction with essential tegument protein ORF44.J Virol. 2014 Jan;88(1):188-201. doi: 10.1128/JVI.02245-13. Epub 2013 Oct 23. J Virol. 2014. PMID: 24155375 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

