Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury
- PMID: 17978027
- PMCID: PMC2562788
- DOI: 10.1523/JNEUROSCI.3647-07.2007
Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury
Abstract
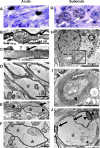
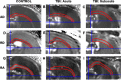
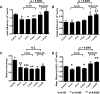
Traumatic axonal injury (TAI) may contribute greatly to neurological impairments after traumatic brain injury, but it is difficult to assess with conventional imaging. We quantitatively compared diffusion tensor imaging (DTI) signal abnormalities with histological and electron microscopic characteristics of pericontusional TAI in a mouse model. Two DTI parameters, relative anisotropy and axial diffusivity, were significantly reduced 6 h to 4 d after trauma, corresponding to relatively isolated axonal injury. One to 4 weeks after trauma, relative anisotropy remained decreased, whereas axial diffusivity "pseudo-normalized" and radial diffusivity increased. These changes corresponded to demyelination, edema, and persistent axonal injury. At every time point, DTI was more sensitive to injury than conventional magnetic resonance imaging, and relative anisotropy distinguished injured from control mice with no overlap between groups. Remarkably, DTI changes strongly predicted the approximate time since trauma. These results provide an important validation of DTI for pericontusional TAI and suggest novel clinical and forensic applications.
Figures

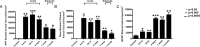
References
-
- Adams H, Mitchell DE, Graham DI, Doyle D. Diffuse brain damage of immediate impact type. Its relationship to “primary brain-stem damage” in head injury. Brain. 1977;100:489–502. - PubMed
-
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. - PubMed
-
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. - PubMed
-
- Albensi BC, Knoblach SM, Chew BG, O'Reilly MP, Faden AI, Pekar JJ. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp Neurol. 2000;162:61–72. - PubMed
-
- Alsop DC, Murai H, Detre JA, McIntosh TK, Smith DH. Detection of acute pathologic changes following experimental traumatic brain injury using diffusion-weighted magnetic resonance imaging. J Neurotrauma. 1996;13:515–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
