Topographic organization in and near human visual area V4
- PMID: 17978030
- PMCID: PMC6673353
- DOI: 10.1523/JNEUROSCI.2991-07.2007
Topographic organization in and near human visual area V4
Abstract
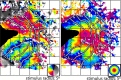
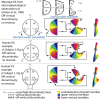
The existence and location of a human counterpart of macaque visual area V4 are disputed. To resolve this issue, we used functional magnetic resonance imaging to obtain topographic maps from human subjects, using visual stimuli and tasks designed to maximize accuracy of topographic maps of the fovea and parafovea and to measure the effects of attention on topographic maps. We identified multiple topographic transitions, each clearly visible in > or = 75% of the maps, that we interpret as boundaries of distinct cortical regions. We call two of these regions dorsal V4 and ventral V4 (together comprising human area V4) because they share several defining characteristics with the macaque regions V4d and V4v (which together comprise macaque area V4). Ventral V4 is adjacent to V3v, and dorsal V4 is adjacent to parafoveal V3d. Ventral V4 and dorsal V4 meet in the foveal confluence shared by V1, V2, and V3. Ventral V4 and dorsal V4 represent complementary regions of the visual field, because ventral V4 represents the upper field and a subregion of the lower field, whereas dorsal V4 represents lower-field locations that are not represented by ventral V4. Finally, attentional modulation of spatial tuning is similar across dorsal and ventral V4, but attention has a smaller effect in V3d and V3v and a larger effect in a neighboring lateral occipital region.
Figures









References
-
- Allman JM, Kaas JH. The organization of the second visual area (VII) in the owl monkey: a second order transformation of the visual hemifield. Brain Res. 1974;76:247–265. - PubMed
-
- Boussaoud D, Ungerleider LG, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. - PubMed
-
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. - PubMed
-
- Brefczynski JA, DeYoe EA. A physiological correlate of the “spotlight” of visual attention. Nat Neurosci. 1999;2:370–374. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
