Neural stem cells improve memory in an inducible mouse model of neuronal loss
- PMID: 17978032
- PMCID: PMC6673368
- DOI: 10.1523/JNEUROSCI.1627-07.2007
Neural stem cells improve memory in an inducible mouse model of neuronal loss
Abstract
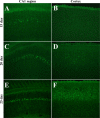
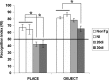
Neuronal loss is a major pathological outcome of many common neurological disorders, including ischemia, traumatic brain injury, and Alzheimer disease. Stem cell-based approaches have received considerable attention as a potential means of treatment, although it remains to be determined whether stem cells can ameliorate memory dysfunction, a devastating component of these disorders. We generated a transgenic mouse model in which the tetracycline-off system is used to regulate expression of diphtheria toxin A chain. After induction, we find progressive neuronal loss primarily within the hippocampus, leading to specific impairments in memory. We find that neural stem cells transplanted into the brain after neuronal ablation survive, migrate, differentiate and, most significantly, improve memory. These results show that stem cells may have therapeutic value in diseases and conditions that result in memory loss.
Figures




Similar articles
-
Impact of hippocampal neuronal ablation on neurogenesis and cognition in the aged brain.Neuroscience. 2014 Feb 14;259:214-22. doi: 10.1016/j.neuroscience.2013.11.054. Epub 2013 Dec 4. Neuroscience. 2014. PMID: 24316470 Free PMC article.
-
Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory.J Neurosci. 2011 Jul 6;31(27):9933-44. doi: 10.1523/JNEUROSCI.1062-11.2011. J Neurosci. 2011. PMID: 21734285 Free PMC article.
-
Grafted neural progenitors migrate and form neurons after experimental traumatic brain injury.Restor Neurol Neurosci. 2009;27(4):323-34. doi: 10.3233/RNN-2009-0481. Restor Neurol Neurosci. 2009. PMID: 19738325
-
Adult neural stem cells from the mouse subventricular zone are limited in migratory ability compared to progenitor cells of similar origin.Neuroscience. 2004;128(4):807-17. doi: 10.1016/j.neuroscience.2004.07.031. Neuroscience. 2004. PMID: 15464288
-
Stem cell therapy for Alzheimer's disease.CNS Neurol Disord Drug Targets. 2011 Jun;10(4):459-85. doi: 10.2174/187152711795563976. CNS Neurol Disord Drug Targets. 2011. PMID: 21495961 Review.
Cited by
-
Autologous skin-derived neural precursor cell therapy reverses canine Alzheimer dementia-like syndrome in a proof of concept veterinary trial.Stem Cell Res Ther. 2022 Jun 17;13(1):261. doi: 10.1186/s13287-022-02933-w. Stem Cell Res Ther. 2022. PMID: 35715872 Free PMC article.
-
In vivo optical signatures of neuronal death in a mouse model of Alzheimer's disease.Lasers Surg Med. 2014 Jan;46(1):27-33. doi: 10.1002/lsm.22206. Epub 2013 Nov 28. Lasers Surg Med. 2014. PMID: 24284732 Free PMC article.
-
Age-Dependent Remarkable Regenerative Potential of the Dentate Gyrus Provided by Intrinsic Stem Cells.J Neurosci. 2020 Jan 29;40(5):974-995. doi: 10.1523/JNEUROSCI.1010-19.2019. Epub 2020 Jan 20. J Neurosci. 2020. PMID: 31959697 Free PMC article.
-
Inducing sterile pyramidal neuronal death in mice to model distinct aspects of gray matter encephalitis.Acta Neuropathol Commun. 2021 Jul 2;9(1):121. doi: 10.1186/s40478-021-01214-6. Acta Neuropathol Commun. 2021. PMID: 34215338 Free PMC article.
-
Cell-mediated neuroprotection in a mouse model of human tauopathy.J Neurosci. 2010 Jul 28;30(30):9973-83. doi: 10.1523/JNEUROSCI.0834-10.2010. J Neurosci. 2010. PMID: 20668182 Free PMC article.
References
-
- Auerbach JM, Eiden MV, McKay RD. Transplanted CNS stem cells form functional synapses in vivo. Eur J Neurosci. 2000;12:1696–1704. - PubMed
-
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. - PubMed
-
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. - PubMed
-
- Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
