Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation
- PMID: 17978049
- PMCID: PMC6673384
- DOI: 10.1523/JNEUROSCI.1109-07.2007
Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation
Abstract
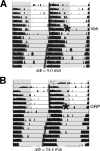
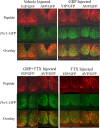
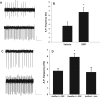
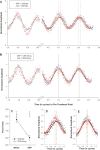
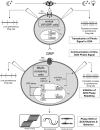
Circadian rhythmicity in the primary mammalian circadian pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus, is maintained by transcriptional and translational feedback loops among circadian clock genes. Photic resetting of the SCN pacemaker involves induction of the clock genes Period1 (Per1) and Period2 (Per2) and communication among distinct cell populations. Gastrin-releasing peptide (GRP) is localized to the SCN ventral retinorecipient zone, from where it may communicate photic resetting signals within the SCN network. Here, we tested the putative role of GRP as an intra-SCN light signal at the behavioral and cellular levels, and we also tested whether GRP actions are dependent on activation of the cAMP response element-binding protein (CREB) pathway and Per1. In vivo microinjections of GRP to the SCN regions of Per1::green fluorescent protein (GFP) mice during the late night induced Per1::GFP throughout the SCN, including a limited population of arginine vasopressin-immunoreactive (AVP-IR) neurons. Blocking spike-mediated communication with tetrodotoxin did not disrupt overall Per1::GFP induction but did reduce induction within AVP-IR neurons. In vitro GRP application resulted in persistent increases in the spike frequency of Per1::GFP-induced neurons. Blocking endogenous Per1 with antisense oligodeoxynucleotides inhibited GRP-induced increases in spike frequency. Furthermore, inhibition of CREB-mediated gene activation with decoy oligonucleotides blocked GRP-induced phase shifts of PER2::luciferase rhythms in SCN slices. Altogether, these results indicate that GRP communicates phase resetting signals within the SCN network via both spike-dependent and spike-independent mechanisms, and that activation of the CREB pathway and Per1 are key steps in mediating downstream events in GRP resetting of SCN neurons.
Figures
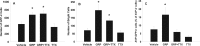
Similar articles
-
Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons.J Biol Rhythms. 2011 Apr;26(2):99-106. doi: 10.1177/0748730410396678. J Biol Rhythms. 2011. PMID: 21454290 Free PMC article.
-
Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons.J Neurosci. 2003 Feb 15;23(4):1441-50. doi: 10.1523/JNEUROSCI.23-04-01441.2003. J Neurosci. 2003. PMID: 12598633 Free PMC article.
-
Roles of light and serotonin in the regulation of gastrin-releasing peptide and arginine vasopressin output in the hamster SCN circadian clock.Eur J Neurosci. 2010 Oct;32(7):1170-9. doi: 10.1111/j.1460-9568.2010.07374.x. Epub 2010 Aug 22. Eur J Neurosci. 2010. PMID: 20731711 Free PMC article.
-
Encoding the ins and outs of circadian pacemaking.J Biol Rhythms. 2006 Dec;21(6):470-81. doi: 10.1177/0748730406294316. J Biol Rhythms. 2006. PMID: 17107937 Review.
-
Sex differences in circadian timing systems: implications for disease.Front Neuroendocrinol. 2014 Jan;35(1):111-39. doi: 10.1016/j.yfrne.2013.11.003. Epub 2013 Nov 25. Front Neuroendocrinol. 2014. PMID: 24287074 Free PMC article. Review.
Cited by
-
Facilitation of the inhibitory transmission by gastrin-releasing peptide in the anterior cingulate cortex.Mol Pain. 2010 Sep 13;6:52. doi: 10.1186/1744-8069-6-52. Mol Pain. 2010. PMID: 20836873 Free PMC article.
-
An LHX1-Regulated Transcriptional Network Controls Sleep/Wake Coupling and Thermal Resistance of the Central Circadian Clockworks.Curr Biol. 2017 Jan 9;27(1):128-136. doi: 10.1016/j.cub.2016.11.008. Epub 2016 Dec 22. Curr Biol. 2017. PMID: 28017605 Free PMC article.
-
Network-mediated encoding of circadian time: the suprachiasmatic nucleus (SCN) from genes to neurons to circuits, and back.J Neurosci. 2014 Nov 12;34(46):15192-9. doi: 10.1523/JNEUROSCI.3233-14.2014. J Neurosci. 2014. PMID: 25392488 Free PMC article. Review.
-
Circadian gating of neuronal functionality: a basis for iterative metaplasticity.Front Syst Neurosci. 2014 Sep 19;8:164. doi: 10.3389/fnsys.2014.00164. eCollection 2014. Front Syst Neurosci. 2014. PMID: 25285070 Free PMC article. Review.
-
Light-regulated translational control of circadian behavior by eIF4E phosphorylation.Nat Neurosci. 2015 Jun;18(6):855-62. doi: 10.1038/nn.4010. Epub 2015 Apr 27. Nat Neurosci. 2015. PMID: 25915475 Free PMC article.
References
-
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. - PubMed
-
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol. 2002;61:26–34. - PubMed
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. - PMC - PubMed
-
- Albers HE, Gillespie CF, Babagbemi TO, Huhman KL. Analysis of the phase shifting effects of gastrin releasing peptide when microinjected into the suprachiasmatic region. Neurosci Lett. 1995;191:63–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
