Cognitive signals in the primate motor thalamus predict saccade timing
- PMID: 17978052
- PMCID: PMC6673367
- DOI: 10.1523/JNEUROSCI.1873-07.2007
Cognitive signals in the primate motor thalamus predict saccade timing
Abstract
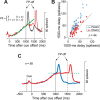
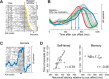
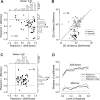
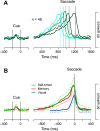
We often generate movements without any external event that immediately triggers them. How the brain decides the timing of self-initiated movements remains unclear. Previous studies suggest that the basal ganglia-thalamocortical pathways play this role, but the subcortical signals that determine movement timing have not been identified. The present study reports that a subset of thalamic neurons predicts the timing of self-initiated saccadic eye movements. When monkeys made a saccade in response to the fixation point (FP) offset in the traditional memory saccade task, neurons in the ventrolateral and the ventroanterior nuclei of the thalamus exhibited a gradual buildup of activity that peaked around the most probable time of the FP offset; however, neither the timing nor the magnitude of neuronal activity correlated with saccade latencies, suggesting that the brain is unlikely to have used this information to decide the times of saccades in the traditional memory saccade task. In contrast, when monkeys were required to make a self-timed saccade within a fixed time interval after an external cue, the same neurons again exhibited a strong buildup of activity that preceded saccades by several hundred milliseconds, showing a close correlation between the times of neuronal activity and the times of self-initiated saccades. The results suggest that neurons in the motor thalamus carry subjective time information, which is used by cortical networks to determine the timing of self-initiated saccades.
Figures
Similar articles
-
Cerebellar Roles in Self-Timing for Sub- and Supra-Second Intervals.J Neurosci. 2017 Mar 29;37(13):3511-3522. doi: 10.1523/JNEUROSCI.2221-16.2017. Epub 2017 Feb 27. J Neurosci. 2017. PMID: 28242799 Free PMC article.
-
Inactivation of the central thalamus delays self-timed saccades.Nat Neurosci. 2006 Jan;9(1):20-2. doi: 10.1038/nn1617. Epub 2005 Dec 11. Nat Neurosci. 2006. PMID: 16341209
-
Striatal dopamine modulates timing of self-initiated saccades.Neuroscience. 2016 Nov 19;337:131-142. doi: 10.1016/j.neuroscience.2016.09.006. Epub 2016 Sep 17. Neuroscience. 2016. PMID: 27651148
-
[Functional analysis of the thalamocortical pathways in eye movements].Brain Nerve. 2011 Aug;63(8):871-7. Brain Nerve. 2011. PMID: 21817178 Review. Japanese.
-
Temporal Information Processing in the Cerebellum and Basal Ganglia.Adv Exp Med Biol. 2024;1455:95-116. doi: 10.1007/978-3-031-60183-5_6. Adv Exp Med Biol. 2024. PMID: 38918348 Review.
Cited by
-
Believer-Skeptic Meets Actor-Critic: Rethinking the Role of Basal Ganglia Pathways during Decision-Making and Reinforcement Learning.Front Neurosci. 2016 Mar 24;10:106. doi: 10.3389/fnins.2016.00106. eCollection 2016. Front Neurosci. 2016. PMID: 27047328 Free PMC article.
-
Activity map of a cortico-cerebellar loop underlying motor planning.Nat Neurosci. 2023 Nov;26(11):1916-1928. doi: 10.1038/s41593-023-01453-x. Epub 2023 Oct 9. Nat Neurosci. 2023. PMID: 37814026 Free PMC article.
-
The Interactions of Temporal and Sensory Representations in the Basal Ganglia.Adv Exp Med Biol. 2024;1455:141-158. doi: 10.1007/978-3-031-60183-5_8. Adv Exp Med Biol. 2024. PMID: 38918350 Review.
-
Correlation between Pupil Size and Subjective Passage of Time in Non-Human Primates.J Neurosci. 2016 Nov 2;36(44):11331-11337. doi: 10.1523/JNEUROSCI.2533-16.2016. J Neurosci. 2016. PMID: 27807173 Free PMC article.
-
Cognitive control of movement via the cerebellar-recipient thalamus.Front Syst Neurosci. 2013 Oct 1;7:56. doi: 10.3389/fnsys.2013.00056. Front Syst Neurosci. 2013. PMID: 24101896 Free PMC article. Review.
References
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Anderson ME, Turner RS. Activity of neurons in cerebellar-receiving and pallidal-receiving areas of the thalamus of the behaving monkey. J Neurophysiol. 1991;66:879–893. - PubMed
-
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. - PubMed
-
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. - PubMed
-
- DeVito JL, Anderson ME. An autoradiographic study of efferent connections of the globus pallidus in Macaca mulatta. Exp Brain Res. 1982;46:107–111. - PubMed
