The structure of the gamma-tubulin small complex: implications of its architecture and flexibility for microtubule nucleation
- PMID: 17978090
- PMCID: PMC2174199
- DOI: 10.1091/mbc.e07-09-0879
The structure of the gamma-tubulin small complex: implications of its architecture and flexibility for microtubule nucleation
Abstract
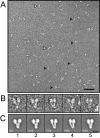
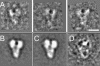
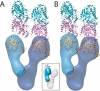
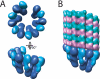
The gamma-tubulin small complex (gamma-TuSC) is an evolutionarily conserved heterotetramer essential for microtubule nucleation. We have determined the structure of the Saccharomyces cerevisiae gamma-TuSC at 25-A resolution by electron microscopy. gamma-TuSC is Y-shaped, with an elongated body connected to two arms. Gold labeling showed that the two gamma-tubulins are located in lobes at the ends of the arms, and the relative orientations of the other gamma-TuSC components were determined by in vivo FRET. The structures of different subpopulations of gamma-TuSC indicate flexibility in the connection between a mobile arm and the rest of the complex, resulting in variation of the relative positions and orientations of the gamma-tubulins. In all of the structures, the gamma-tubulins are distinctly separated, a configuration incompatible with the microtubule lattice. The separation of the gamma-tubulins in isolated gamma-TuSC likely plays a role in suppressing its intrinsic microtubule-nucleating activity, which is relatively weak until the gamma-TuSC is incorporated into higher order complexes or localized to microtubule-organizing centers. We propose that further movement of the mobile arm is required to bring the gamma-tubulins together in microtubule-like interactions, and provide a template for microtubule growth.
Figures
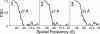
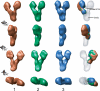


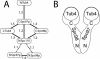
Similar articles
-
Microtubule nucleating gamma-TuSC assembles structures with 13-fold microtubule-like symmetry.Nature. 2010 Aug 12;466(7308):879-82. doi: 10.1038/nature09207. Epub 2010 Jul 14. Nature. 2010. PMID: 20631709 Free PMC article.
-
Localization and orientation of the gamma-tubulin small complex components using protein tags as labels for single particle EM.J Struct Biol. 2009 Dec;168(3):571-4. doi: 10.1016/j.jsb.2009.08.012. Epub 2009 Aug 31. J Struct Biol. 2009. PMID: 19723581 Free PMC article.
-
The microtubule polymerase Stu2 promotes oligomerization of the γ-TuSC for cytoplasmic microtubule nucleation.Elife. 2018 Sep 17;7:e39932. doi: 10.7554/eLife.39932. Elife. 2018. PMID: 30222109 Free PMC article.
-
Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence.Trends Cell Biol. 2015 May;25(5):296-307. doi: 10.1016/j.tcb.2014.12.002. Epub 2014 Dec 24. Trends Cell Biol. 2015. PMID: 25544667 Review.
-
Acentrosomal microtubule nucleation in higher plants.Int Rev Cytol. 2002;220:257-89. doi: 10.1016/s0074-7696(02)20008-x. Int Rev Cytol. 2002. PMID: 12224551 Review.
Cited by
-
CDK5RAP2 stimulates microtubule nucleation by the gamma-tubulin ring complex.J Cell Biol. 2010 Dec 13;191(6):1089-95. doi: 10.1083/jcb.201007030. Epub 2010 Dec 6. J Cell Biol. 2010. PMID: 21135143 Free PMC article.
-
Assembly and regulation of γ-tubulin complexes.Open Biol. 2018 Mar;8(3):170266. doi: 10.1098/rsob.170266. Open Biol. 2018. PMID: 29514869 Free PMC article. Review.
-
Regulation of microtubule nucleation mediated by γ-tubulin complexes.Protoplasma. 2017 May;254(3):1187-1199. doi: 10.1007/s00709-016-1070-z. Epub 2017 Jan 10. Protoplasma. 2017. PMID: 28074286 Review.
-
The molecular architecture of the yeast spindle pole body core determined by Bayesian integrative modeling.Mol Biol Cell. 2017 Nov 7;28(23):3298-3314. doi: 10.1091/mbc.E17-06-0397. Epub 2017 Aug 16. Mol Biol Cell. 2017. PMID: 28814505 Free PMC article.
-
Laterally attached kinetochores recruit the checkpoint protein Bub1, but satisfy the spindle checkpoint.Cell Cycle. 2010 Sep 1;9(17):3619-28. doi: 10.4161/cc.9.17.12907. Cell Cycle. 2010. PMID: 20928940 Free PMC article.
References
-
- Aldaz H., Rice L. M., Stearns T., Agard D. A. Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature. 2005;435:523–527. - PubMed
-
- Bottcher B., Wynne S. A., Crowther R. A. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. - PubMed
-
- Byers B., Shriver K., Goetsch L. The role of spindle pole bodies and modified microtubule ends in the initiation of microtubule assembly in Saccharomyces cerevisiae. J. Cell Sci. 1978;30:331–352. - PubMed
-
- Erickson H. P. Gamma-tubulin nucleation: template or protofilament? Nat. Cell Biol. 2000;2:E93–E96. - PubMed
-
- Frank J., Radermacher M., Penczek P., Zhu J., Li Y., Ladjadj M., Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 1996;116:190–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

