Precursor oxidation by Mia40 and Erv1 promotes vectorial transport of proteins into the mitochondrial intermembrane space
- PMID: 17978092
- PMCID: PMC2174169
- DOI: 10.1091/mbc.e07-08-0814
Precursor oxidation by Mia40 and Erv1 promotes vectorial transport of proteins into the mitochondrial intermembrane space
Erratum in
- Mol Biol Cell. 2014 Apr;25(8):1408
Abstract
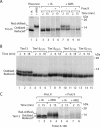
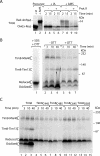
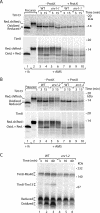
The mitochondrial intermembrane space contains chaperone complexes that guide hydrophobic precursor proteins through this aqueous compartment. The chaperones consist of hetero-oligomeric complexes of small Tim proteins with conserved cysteine residues. The precursors of small Tim proteins are synthesized in the cytosol. Import of the precursors requires the essential intermembrane space proteins Mia40 and Erv1 that were proposed to form a relay for disulfide formation in the precursor proteins. However, experimental evidence for a role of Mia40 and Erv1 in the oxidation of intermembrane space precursors has been lacking. We have established a system to directly monitor the oxidation of precursors during import into mitochondria and dissected distinct steps of the import process. Reduced precursors bind to Mia40 during translocation into mitochondria. Both Mia40 and Erv1 are required for formation of oxidized monomers of the precursors that subsequently assemble into oligomeric complexes. Whereas the reduced precursors can diffuse back into the cytosol, the oxidized precursors are retained in the intermembrane space. Thus, oxidation driven by Mia40 and Erv1 determines vectorial transport of the precursors into the mitochondrial intermembrane space.
Figures
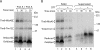


References
-
- Allen S., Balabanidou V., Sideris D. P., Lisowsky T., Tokatlidis K. Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J. Mol. Biol. 2005;353:937–944. - PubMed
-
- Allen S., Lu H., Thornton D., Tokatlidis K. Juxtaposition of the two distal Cx3C motifs via intrachain disulfide bonding is essential for the folding of Tim10. J. Biol. Chem. 2003;278:38505–38513. - PubMed
-
- Bohnert M., Pfanner N., van der Laan M. A dynamic machinery for import of mitochondrial precursor proteins. FEBS Lett. 2007;581:2802–2810. - PubMed
-
- Chacinska A., et al. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell. 2005;120:817–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

