Distinct roles for two Galpha-Gbeta interfaces in cell polarity control by a yeast heterotrimeric G protein
- PMID: 17978098
- PMCID: PMC2174166
- DOI: 10.1091/mbc.e07-04-0385
Distinct roles for two Galpha-Gbeta interfaces in cell polarity control by a yeast heterotrimeric G protein
Abstract
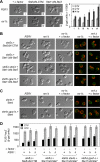
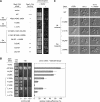
Saccharomyces cerevisiae mating pheromones trigger dissociation of a heterotrimeric G protein (Galphabetagamma) into Galpha-guanosine triphosphate (GTP) and Gbetagamma. The Gbetagamma dimer regulates both mitogen-activated protein (MAP) kinase cascade signaling and cell polarization. Here, by independently activating the MAP kinase pathway, we studied the polarity role of Gbetagamma in isolation from its signaling role. MAP kinase signaling alone could induce cell asymmetry but not directional growth. Surprisingly, active Gbetagamma, either alone or with Galpha-GTP, could not organize a persistent polarization axis. Instead, following pheromone gradients (chemotropism) or directional growth without pheromone gradients (de novo polarization) required an intact receptor-Galphabetagamma module and GTP hydrolysis by Galpha. Our results indicate that chemoattractant-induced cell polarization requires continuous receptor-Galphabetagamma communication but not modulation of MAP kinase signaling. To explore regulation of Gbetagamma by Galpha, we mutated Gbeta residues in two structurally distinct Galpha-Gbeta binding interfaces. Polarity control was disrupted only by mutations in the N-terminal interface, and not the Switch interface. Incorporation of these mutations into a Gbeta-Galpha fusion protein, which enforces subunit proximity, revealed that Switch interface dissociation regulates signaling, whereas the N-terminal interface may govern receptor-Galphabetagamma coupling. These findings raise the possibility that the Galphabetagamma heterotrimer can function in a partially dissociated state, tethered by the N-terminal interface.
Figures







References
-
- Apanovitch D. M., Iiri T., Karasawa T., Bourne H. R., Dohlman H. G. Second site suppressor mutations of a GTPase-deficient G-protein α-subunit. Selective inhibition of Gβγ-mediated signaling. J. Biol. Chem. 1998;273:28597–28602. - PubMed
-
- Arkowitz R. A. Responding to attraction: chemotaxis and chemotropism in Dictyostelium and yeast. Trends Cell Biol. 1999;9:20–27. - PubMed
-
- Ayscough K. R., Drubin D. G. A role for the yeast actin cytoskeleton in pheromone receptor clustering and signalling. Curr. Biol. 1998;8:927–930. - PubMed
-
- Bagorda A., Mihaylov V. A., Parent C. A. Chemotaxis: moving forward and holding on to the past. Thromb. Haemost. 2006;95:12–21. - PubMed
-
- Bar E. E., Ellicott A. T., Stone D. E. Gbetagamma recruits Rho1 to the site of polarized growth during mating in budding yeast. J. Biol. Chem. 2003;278:21798–21804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

