Application of high-throughput screening to identify a novel alphaIIb-specific small- molecule inhibitor of alphaIIbbeta3-mediated platelet interaction with fibrinogen
- PMID: 17978171
- PMCID: PMC2214768
- DOI: 10.1182/blood-2007-08-105544
Application of high-throughput screening to identify a novel alphaIIb-specific small- molecule inhibitor of alphaIIbbeta3-mediated platelet interaction with fibrinogen
Abstract
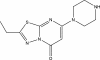
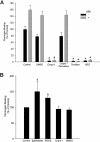
Small-molecule alphaIIbbeta3 antagonists competitively block ligand binding by spanning between the D224 in alphaIIb and the MIDAS metal ion in beta3. They variably induce conformational changes in the receptor, which may have undesirable consequences. To identify alphaIIbbeta3 antagonists with novel structures, we tested 33 264 small molecules for their ability to inhibit the adhesion of washed platelets to immobilized fibrinogen at 16 muM. A total of 102 compounds demonstrated 50% or more inhibition, and one of these (compound 1, 265 g/mol) inhibited ADP-induced platelet aggregation (IC(50): 13+/- 5 muM), the binding of soluble fibrinogen to platelets induced by mAb AP5, and the binding of soluble fibrinogen and a cyclic RGD peptide to purified alphaIIbbeta3. Compound 1 did not affect the function of GPIb, alpha2beta1, or the other beta3 family receptor alphaVbeta3. Molecular docking simulations suggest that compound 1 interacts with alphaIIb but not beta3. Compound 1 induced partial exposure of an alphaIIb ligand-induced binding site (LIBS), but did not induce exposure of 2 beta3 LIBS. Transient exposure of purified alphaIIbbeta3 to eptifibatide, but not compound 1, enhanced fibrinogen binding ("priming"). Compound 1 provides a prototype for small molecule selective inhibition of alphaIIbbeta3, without receptor priming, via targeting alphaIIb.
Figures





References
-
- Phillips DR, Charo IF, Scarborough RM. GPIIb-IIIa: the responsive integrin. Cell. 1991;65:359–362. - PubMed
-
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–516. - PubMed
-
- Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. - PubMed
-
- Coller BS. Interaction of normal, thrombasthenic, and Bernard-Soulier platelets with immobilized fibrinogen: defective platelet-fibrinogen interaction in thrombasthenia. Blood. 1980;55:169–178. - PubMed
-
- Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

