Duplication and segregation of the actin (MreB) cytoskeleton during the prokaryotic cell cycle
- PMID: 17978175
- PMCID: PMC2077029
- DOI: 10.1073/pnas.0708739104
Duplication and segregation of the actin (MreB) cytoskeleton during the prokaryotic cell cycle
Abstract
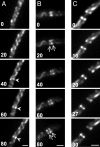
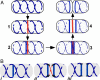
The bacterial actin homolog MreB exists as a single-copy helical cytoskeletal structure that extends between the two poles of rod-shaped bacteria. In this study, we show that equipartition of the MreB cytoskeleton into daughter cells is accomplished by division and segregation of the helical MreB array into two equivalent structures located in opposite halves of the predivisional cell. This process ensures that each daughter cell inherits one copy of the MreB cytoskeleton. The process is triggered by the membrane association of the FtsZ cell division protein. The cytoskeletal division and segregation events occur before and independently of cytokinesis and involve specialized MreB structures that appear to be intermediates in this process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

