In vitro synthesis of the iron-molybdenum cofactor of nitrogenase from iron, sulfur, molybdenum, and homocitrate using purified proteins
- PMID: 17978192
- PMCID: PMC2077076
- DOI: 10.1073/pnas.0703050104
In vitro synthesis of the iron-molybdenum cofactor of nitrogenase from iron, sulfur, molybdenum, and homocitrate using purified proteins
Abstract
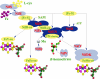
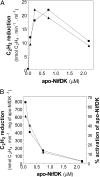
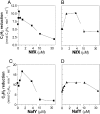
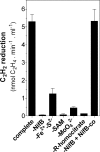
Biological nitrogen fixation, the conversion of atmospheric N2 to NH3, is an essential process in the global biogeochemical cycle of nitrogen that supports life on Earth. Most of the biological nitrogen fixation is catalyzed by the molybdenum nitrogenase, which contains at its active site one of the most complex metal cofactors known to date, the iron-molybdenum cofactor (FeMo-co). FeMo-co is composed of 7Fe, 9S, Mo, R-homocitrate, and one unidentified light atom. Here we demonstrate the complete in vitro synthesis of FeMo-co from Fe(2+), S(2-), MoO4(2-), and R-homocitrate using only purified Nif proteins. This synthesis provides direct biochemical support to the current model of FeMo-co biosynthesis. A minimal in vitro system, containing NifB, NifEN, and NifH proteins, together with Fe(2+), S(2-), MoO4(2-), R-homocitrate, S-adenosyl methionine, and Mg-ATP, is sufficient for the synthesis of FeMo-co and the activation of apo-dinitrogenase under anaerobic-reducing conditions. This in vitro system also provides a biochemical approach to further study the function of accessory proteins involved in nitrogenase maturation (as shown here for NifX and NafY). The significance of these findings in the understanding of the complete FeMo-co biosynthetic pathway and in the study of other complex Fe-S cluster biosyntheses is discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Metal trafficking for nitrogen fixation: NifQ donates molybdenum to NifEN/NifH for the biosynthesis of the nitrogenase FeMo-cofactor.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11679-84. doi: 10.1073/pnas.0803576105. Epub 2008 Aug 12. Proc Natl Acad Sci U S A. 2008. PMID: 18697927 Free PMC article.
-
Nitrogenase cofactor biosynthesis using proteins produced in mitochondria of Saccharomyces cerevisiae.mBio. 2024 Feb 14;15(2):e0308823. doi: 10.1128/mbio.03088-23. Epub 2023 Dec 21. mBio. 2024. PMID: 38126768 Free PMC article.
-
NifX and NifEN exchange NifB cofactor and the VK-cluster, a newly isolated intermediate of the iron-molybdenum cofactor biosynthetic pathway.Mol Microbiol. 2007 Jan;63(1):177-92. doi: 10.1111/j.1365-2958.2006.05514.x. Epub 2006 Dec 5. Mol Microbiol. 2007. PMID: 17163967
-
Biosynthesis of the iron-molybdenum cofactor of nitrogenase.Crit Rev Biotechnol. 1994;14(3):225-49. doi: 10.3109/07388554409079834. Crit Rev Biotechnol. 1994. PMID: 7954845 Review.
-
Biosynthesis of the iron-molybdenum cofactor of nitrogenase.Biofactors. 1988 Oct;1(3):199-205. Biofactors. 1988. PMID: 3076773 Review.
Cited by
-
Biosynthesis of the nitrogenase active-site cofactor precursor NifB-co in Saccharomyces cerevisiae.Proc Natl Acad Sci U S A. 2019 Dec 10;116(50):25078-25086. doi: 10.1073/pnas.1904903116. Epub 2019 Nov 25. Proc Natl Acad Sci U S A. 2019. PMID: 31767756 Free PMC article.
-
Methanogen homoaconitase catalyzes both hydrolyase reactions in coenzyme B biosynthesis.J Biol Chem. 2008 Oct 24;283(43):28888-96. doi: 10.1074/jbc.M802159200. Epub 2008 Sep 2. J Biol Chem. 2008. PMID: 18765671 Free PMC article.
-
A sterile alpha-motif domain in NafY targets apo-NifDK for iron-molybdenum cofactor delivery via a tethered domain.J Biol Chem. 2011 Feb 25;286(8):6321-8. doi: 10.1074/jbc.M110.168732. Epub 2010 Dec 14. J Biol Chem. 2011. PMID: 21156797 Free PMC article.
-
Natural history of the E1-like superfamily: implication for adenylation, sulfur transfer, and ubiquitin conjugation.Proteins. 2009 Jun;75(4):895-910. doi: 10.1002/prot.22298. Proteins. 2009. PMID: 19089947 Free PMC article.
-
Radical S-adenosylmethionine enzymes.Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

