The efficacy and tolerability of nebivolol in hypertensive African American patients
- PMID: 17978594
- PMCID: PMC8110055
- DOI: 10.1111/j.1524-6175.2007.07548.x
The efficacy and tolerability of nebivolol in hypertensive African American patients
Abstract
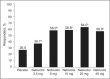
Hypertensive African Americans often respond poorly to beta-blocker monotherapy, compared with whites. There is evidence, however, that suggests that this response may be different if beta-blockers with vasodilating effects are used. This 12-week, multi-center, double-blind, randomized placebo-controlled study assessed the antihypertensive efficacy and safety of nebivolol, a cardioselective, vasodilating beta1-blocker, at doses of 2.5, 5, 10, 20, or 40 mg once daily in 300 African American patients with stage I or II hypertension (mean sitting diastolic blood pressure [SiDBP] > or =95 mm Hg and < or =109 mm Hg). The primary efficacy end point was the baseline-adjusted change in trough mean SiDBP. After 12 weeks, nebivolol significantly reduced least squares mean SiDBP (P< or =.004) at all doses of 5 mg and higher and sitting systolic blood pressure (P< or =.044) at all doses 10 mg and higher, compared with placebo. The drug was safe and well-tolerated, with no significant difference in the incidence of adverse events compared with placebo. Nebivolol monotherapy provides antihypertensive efficacy, with few significant adverse effects, in hypertensive African Americans.
Figures

References
-
- Centers for Disease Control and Prevention (CDC) . Racial/ethnic disparities in prevalence, treatment, and control of hypertension—United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54:7–9. - PubMed
-
- Sowers JR, Ferdinand KC, Bakris GL, et al. Hypertension‐related disease in African Americans: factors underlying disparities in illness and its outcome. Postgrad Med. 2002;112:24–48. - PubMed
-
- Stein CM, Lang CC, Singh I, et al. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks: additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–951. - PubMed
-
- Jones DS, Andrawis NS, Abernethy DR. Impaired endothelial dependent forearm vascular relaxation in black Americans. Clin Pharmacol Ther. 1999;65:408–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

