Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones: synthesis and preliminary QSAR studies
- PMID: 17978766
- PMCID: PMC6149171
- DOI: 10.3390/12102413
Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones: synthesis and preliminary QSAR studies
Abstract
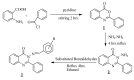
Synthesis of ten 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones is reported. All the compounds contained a common phenyl group at the 2-position, while the substituents on the arylideneamino group were varied. The compounds were investigated for their antimicrobial activity against both Gram-positive (Staphylococcus aureus 6571 and Bacillus subtilis) and Gram-negative bacteria (Escherichia coli K12 and Shigella dysenteriae 6) using a turbidometric assay method. It was found that the incorporation of the 3-arylideneamino substituent enhanced the anti-bacterial activity of the quinazolone system. The preliminary QSAR studies were done using some computer derived property descriptors, calculated values of partition coefficients as well as usual Hammett's sigma constants and the substituent's molar refractivity.
Figures
Similar articles
-
Antibacterial effect of some substituted tricyclic quinazolines and their synthetic precursors.Folia Microbiol (Praha). 1999;44(2):187-90. doi: 10.1007/BF02816240. Folia Microbiol (Praha). 1999. PMID: 10588054
-
Synthesis and QSAR modeling of 2-acetyl-2-ethoxycarbonyl-1-[4(4'-arylazo)-phenyl]-N,N-dimethylaminophenyl aziridines as potential antibacterial agents.Eur J Med Chem. 2009 Jan;44(1):251-9. doi: 10.1016/j.ejmech.2008.02.016. Epub 2008 Feb 29. Eur J Med Chem. 2009. PMID: 18396359
-
Antibacterial efficacy of Rumex nepalensis Spreng. roots.Phytother Res. 2003 May;17(5):558-9. doi: 10.1002/ptr.1162. Phytother Res. 2003. PMID: 12748999
-
Synthesis, characterization, biological evaluation and QSAR studies of 11-p-substituted phenyl-12-phenyl-11a,12-dihydro-11H-indeno[2,1-c][1,5]benzothiazepines as potential antimicrobial agents.Eur J Med Chem. 2012 Nov;57:196-210. doi: 10.1016/j.ejmech.2012.09.003. Epub 2012 Sep 11. Eur J Med Chem. 2012. PMID: 23047232
-
Quinazolines annelated at the N(3)-C(4) bond: Synthesis and biological activity.Eur J Med Chem. 2024 May 5;271:116411. doi: 10.1016/j.ejmech.2024.116411. Epub 2024 Apr 18. Eur J Med Chem. 2024. PMID: 38669910 Review.
Cited by
-
Synthesis and cytotoxic evaluation of some new 3-(2-(2-phenylthiazol-4-yl) ethyl)-quinazolin-4(3H) one derivatives with potential anticancer effects.Res Pharm Sci. 2017 Aug;12(4):290-298. doi: 10.4103/1735-5362.212046. Res Pharm Sci. 2017. PMID: 28855940 Free PMC article.
-
Synthesis and cytotoxicity evaluation of some new 6-nitro derivatives of thiazole-containing 4-(3H)-quinazolinone.Res Pharm Sci. 2016 May-Jun;11(3):210-8. Res Pharm Sci. 2016. PMID: 27499790 Free PMC article.
-
Synthesis, Spectroscopic Characterization, and Time-Dependent DFT Calculations of 1-Methyl-5-phenyl-5H-pyrido[1,2-a]quinazoline-3,6-dione and Its Starting Precursor in Different Solvents.ChemistryOpen. 2018 Oct 12;7(10):814-823. doi: 10.1002/open.201800146. eCollection 2018 Oct. ChemistryOpen. 2018. PMID: 30338205 Free PMC article.
-
Iridium-catalysed direct sulfamidation of quinazolinones.RSC Adv. 2018 Feb 23;8(16):8450-8454. doi: 10.1039/c8ra00524a. eCollection 2018 Feb 23. RSC Adv. 2018. PMID: 35539849 Free PMC article.
-
Antibacterial, antifungal and cytotoxic evaluation of some new 2,3-disubstituted 4(3H)-quinazolinone derivatives.Res Pharm Sci. 2012 Jul;7(3):151-8. Res Pharm Sci. 2012. PMID: 23181093 Free PMC article.
References
-
- Spirkova K., Stankovsky S., Mrvova A., Cipak L. Synthesis and Biological Activity of Some 2-substituted Quinazolin-4-ones. Chem. Pap. 1999;53:272–275.
-
- Alagarsamy V., Giridhar R., Yadav H. R., Revathi R., Rukmani K., De Clercq E. Anti HIV, antibacterial and antifungal activities of some novel1,4-disubstituted-1,2,4-triazolo[4,3a] quinazolin-5(4h)-ones. Indian J. Pharm. Sci. 2006;68:532–535. doi: 10.4103/0250-474X.27840. - DOI
-
- Srivastava V. K., Singh A., Gucati A., Shankar K. Antiparkinsonian agent from qunazonyl thioazolidinones and azetidinones. Indian J. Chem. 1987;26:652–656.
-
- Gupta D. P., Ahmed S., Kumar A., Shankar K. Newer Quinazolinone Derivatives as Anthelmintic Agents. Indian J. Chem. 1988;27:1060–1062.
-
- Jatav V., Mishra P., Kaswa S., Stabes J. P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2007 ? - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical