Evaluating a surrogate endpoint at three levels, with application to vaccine development
- PMID: 17979212
- PMCID: PMC2646675
- DOI: 10.1002/sim.3122
Evaluating a surrogate endpoint at three levels, with application to vaccine development
Abstract
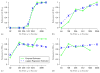
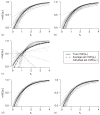
Identification of an immune response to vaccination that reliably predicts protection from clinically significant infection, i.e. an immunological surrogate endpoint, is a primary goal of vaccine research. Using this problem of evaluating an immunological surrogate as an illustration, we describe a hierarchy of three criteria for a valid surrogate endpoint and statistical analysis frameworks for evaluating them. Based on a placebo-controlled vaccine efficacy trial, the first level entails assessing the correlation of an immune response with a study endpoint in the study groups, and the second level entails evaluating an immune response as a surrogate for the study endpoint that can be used for predicting vaccine efficacy for a setting similar to that of the vaccine trial. We show that baseline covariates, innovative study design, and a potential outcomes formulation can be helpful for this assessment. The third level entails validation of a surrogate endpoint via meta-analysis, where the goal is to evaluate how well the immune response can be used to predict vaccine efficacy for new settings (building bridges). A simulated vaccine trial and two example vaccine trials are presented, one supporting that certain anti-influenza antibody levels are an excellent surrogate for influenza illness and another supporting that certain anti-HIV antibody levels are not useful as a surrogate for HIV infection.
Copyright (c) 2007 John Wiley & Sons, Ltd.
Figures


Comment in
-
Comment on 'Evaluating a surrogate endpoint at three levels, with application to vaccine development' by Peter B. Gilbert, Li Qin and Steven G. Self, Statistics in Medicine, DOI: 10.1002/sim.3122.Stat Med. 2008 Dec 20;27(29):6268-70. doi: 10.1002/sim.3425. Stat Med. 2008. PMID: 18767203 No abstract available.
References
-
- Fauci AS, Haynes BF, Pantaleo G. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. - PubMed
-
- Clements-Mann ML. Lessons for AIDS vaccine development from non-AIDS vaccines. AIDS Research and Human Retroviruses. 1998;14(Suppl 3):S197–S203. - PubMed
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nature Immunology. 2004;5:233–236. - PubMed
-
- Siber GR. Methods for estimating serological correlates of protection. Developments in Biological Standardization. 1997;89:283–296. - PubMed
-
- Chan ISF, Shu L, Matthews H, Chan C, Vessey R, Sadoff J, Heyse J. Use of statistical models for evaluating antibody response as a correlate of protection against varicella. Statistics in Medicine. 2002;21:3411–3430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
