Farnesyl diphosphate analogues with omega-bioorthogonal azide and alkyne functional groups for protein farnesyl transferase-catalyzed ligation reactions
- PMID: 17979291
- PMCID: PMC2516946
- DOI: 10.1021/jo7017747
Farnesyl diphosphate analogues with omega-bioorthogonal azide and alkyne functional groups for protein farnesyl transferase-catalyzed ligation reactions
Abstract
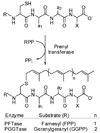
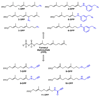
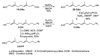
Eleven farnesyl diphosphate analogues, which contained omega-azide or alkyne substituents suitable for bioorthogonal Staudinger and Huisgen [3 + 2] cycloaddition coupling reactions, were synthesized. The analogues were evaluated as substrates for the alkylation of peptide cosubstrates by yeast protein farnesyl transferase. Five of the diphosphates were good alternative substrates for farnesyl diphosphate (FPP). Steady-state kinetic constants were measured for the active compounds, and the products were characterized by HPLC and LC-MS. Two of the analogues gave steady-state kinetic parameters (kcat and Km) very similar to those of the natural substrate.
Figures










Similar articles
-
Photoaffinity analogues of farnesyl pyrophosphate transferable by protein farnesyl transferase.J Am Chem Soc. 2002 Jul 17;124(28):8206-19. doi: 10.1021/ja0124717. J Am Chem Soc. 2002. PMID: 12105898
-
Selective modification of CaaX peptides with ortho-substituted anilinogeranyl lipids by protein farnesyl transferase: competitive substrates and potent inhibitors from a library of farnesyl diphosphate analogues.Biochemistry. 2007 Oct 9;46(40):11310-21. doi: 10.1021/bi700516m. Epub 2007 Sep 14. Biochemistry. 2007. PMID: 17854205
-
Farnesyl diphosphate analogues with aryl moieties are efficient alternate substrates for protein farnesyltransferase.Biochemistry. 2012 Oct 16;51(41):8307-19. doi: 10.1021/bi3011362. Epub 2012 Oct 2. Biochemistry. 2012. PMID: 22989235 Free PMC article.
-
Multifluorinated Aryl Azides for the Development of Improved H2 S Probes, and Fast Strain-promoted Azide-Alkyne Cycloaddition and Staudinger Reactions.Chem Asian J. 2020 May 4;15(9):1420-1429. doi: 10.1002/asia.202000005. Epub 2020 Mar 23. Chem Asian J. 2020. PMID: 32144862 Review.
-
Electrophilic Azides for Materials Synthesis and Chemical Biology.Acc Chem Res. 2020 Apr 21;53(4):937-948. doi: 10.1021/acs.accounts.0c00046. Epub 2020 Mar 24. Acc Chem Res. 2020. PMID: 32207916 Review.
Cited by
-
Simultaneous dual protein labeling using a triorthogonal reagent.J Am Chem Soc. 2013 Nov 6;135(44):16388-96. doi: 10.1021/ja403813b. Epub 2013 Oct 17. J Am Chem Soc. 2013. PMID: 24134212 Free PMC article.
-
Engineering protein farnesyltransferase for enzymatic protein labeling applications.Bioconjug Chem. 2014 Jul 16;25(7):1203-12. doi: 10.1021/bc500240p. Epub 2014 Jul 2. Bioconjug Chem. 2014. PMID: 24946229 Free PMC article.
-
Simultaneous Site-Specific Dual Protein Labeling Using Protein Prenyltransferases.Bioconjug Chem. 2015 Dec 16;26(12):2542-53. doi: 10.1021/acs.bioconjchem.5b00553. Epub 2015 Dec 4. Bioconjug Chem. 2015. PMID: 26561785 Free PMC article.
-
Antitrypanosomal and antileishmanial activity of prenyl-1,2,3-triazoles.Medchemcomm. 2017 May 1;8(5):1015-1021. doi: 10.1039/C7MD00008A. Epub 2017 Mar 10. Medchemcomm. 2017. PMID: 28993794 Free PMC article.
-
Purification of prenylated proteins by affinity chromatography on cyclodextrin-modified agarose.Anal Biochem. 2009 Mar 1;386(1):1-8. doi: 10.1016/j.ab.2008.09.007. Epub 2008 Sep 14. Anal Biochem. 2009. PMID: 18834849 Free PMC article.
References
-
- Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, Bishop WR, Kirschmeier P. J. Biol. Chem. 2000;275:30451–30457. - PubMed
-
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Trends Cell. Biol. 2000;10:147–154. - PubMed
-
- Sebti SM, Hamilton AD. Oncogene. 2000;19:6584–6593. - PubMed
-
- Bell IM. J. Med. Chem. 2004;47:1869–1878. - PubMed
-
- Appels NM, Beijnen JH, Schellens JH. Oncologist. 2005;10:565–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

