The trafficking of natural killer cells
- PMID: 17979846
- PMCID: PMC7165697
- DOI: 10.1111/j.1600-065X.2007.00563.x
The trafficking of natural killer cells
Abstract
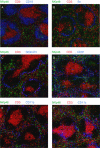
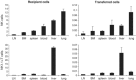
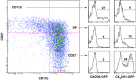
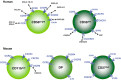
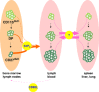
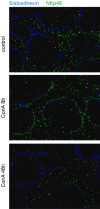
Natural killer (NK) cells are large granular lymphocytes of the innate immune system that participate in the early control of microbial infections and cancer. NK cells can induce the death of autologous cells undergoing various forms of stress, recognizing and providing non-microbial 'danger' signals to the immune system. NK cells are widely distributed in lymphoid and non-lymphoid organs. NK cell precursors originate from the bone marrow and go through a complex maturation process that leads to the acquisition of their effector functions, to changes in their expression of integrins and chemotactic receptors, and to their redistribution from the bone marrow and lymph nodes to blood, spleen, liver, and lung. Here, we describe the tissue localization of NK cells, using NKp46 as an NK cell marker, and review the current knowledge on the mechanisms that govern their trafficking in humans and in mice.
Figures


References
-
- Moretta A, Bottino C, Mingari MC, Biassoni R, Moretta L. What is a natural killer cell? Nat Immunol 2002;3:6–8. - PubMed
-
- Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol 2004;5:996–1002. - PubMed
-
- Cerwenka A, Lanier LL. Ligands for natural killer cell receptors: redundancy or specificity. Immunol Rev 2001;181:158–169. - PubMed
-
- Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 2002;20:217–251. - PubMed
-
- Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science 2004;306:1517–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

