Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses
- PMID: 17979850
- PMCID: PMC7165931
- DOI: 10.1111/j.1600-065X.2007.00564.x
Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses
Abstract
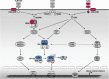
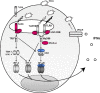
Five of the 10 human Toll-like receptors (TLRs) (TLR3, TLR4, TLR7, TLR8, and TLR9), and four of the 12 mouse TLRs (TLR3, TLR4, TLR7, TLR9) can trigger interferon (IFN)-alpha, IFN-beta, and IFN-lambda, which are critical for antiviral immunity. Moreover, TLR3, TLR7, TLR8, and TLR9 differ from TLR4 in two particularly important ways for antiviral immunity: they can be activated by nucleic acid agonists mimicking compounds produced during the viral cycle, and they are typically present within the cell, along the endocytic pathway, where they sense viral products in the intraluminal space. Investigations in mice have demonstrated that the TLR7/9-IFN and TLR3-IFN pathways are different and critical for protective immunity to various experimental viral infections. Investigations in humans with interleukin-1 receptor-associated kinase-4 (IRAK-4) deficiency (unresponsive to TLR7, TLR8, and TLR9), UNC-93B deficiency (unresponsive to TLR3, TLR7, TLR8, and TLR9), and TLR3 deficiency have recently shed light on the role of these two pathways in antiviral immunity in natural conditions. UNC-93B- and TLR3-deficient patients appear to be specifically prone to herpes simplex virus 1 (HSV-1) encephalitis, although clinical penetrance is incomplete, whereas IRAK-4-deficient patients appear to be normally resistant to most viruses, including HSV-1. These experiments of nature suggest that the TLR7-, TLR8-, and TLR9-dependent induction of IFN-alpha, IFN-beta, and IFN-lambda is largely redundant in human antiviral immunity, whereas the TLR3-dependent induction of IFN-alpha, IFN-beta, and IFN-lambda is critical for primary immunity to HSV-1 in the central nervous system in children but redundant for immunity to most other viral infections.
Figures
Similar articles
-
Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells.Nature. 2012 Nov 29;491(7426):769-73. doi: 10.1038/nature11583. Epub 2012 Oct 28. Nature. 2012. PMID: 23103873 Free PMC article.
-
Interleukin receptor-associated kinase-4 deficiency impairs Toll-like receptor-dependent innate antiviral immune responses.J Allergy Clin Immunol. 2006 Dec;118(6):1357-62. doi: 10.1016/j.jaci.2006.08.006. Epub 2006 Sep 25. J Allergy Clin Immunol. 2006. PMID: 17157666
-
Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses.Immunity. 2005 Nov;23(5):465-78. doi: 10.1016/j.immuni.2005.09.016. Immunity. 2005. PMID: 16286015 Free PMC article.
-
Mendelian predisposition to herpes simplex encephalitis.Handb Clin Neurol. 2013;112:1091-7. doi: 10.1016/B978-0-444-52910-7.00027-1. Handb Clin Neurol. 2013. PMID: 23622315 Review.
-
TLR3 immunity to infection in mice and humans.Curr Opin Immunol. 2013 Feb;25(1):19-33. doi: 10.1016/j.coi.2012.11.001. Epub 2013 Jan 3. Curr Opin Immunol. 2013. PMID: 23290562 Free PMC article. Review.
Cited by
-
Stat2 loss leads to cytokine-independent, cell-mediated lethality in LPS-induced sepsis.Proc Natl Acad Sci U S A. 2013 May 21;110(21):8656-61. doi: 10.1073/pnas.1221652110. Epub 2013 May 7. Proc Natl Acad Sci U S A. 2013. PMID: 23653476 Free PMC article.
-
Post-COVID-19 HSV encephalitis: a review.QJM. 2022 Apr 20;115(4):222-227. doi: 10.1093/qjmed/hcac060. QJM. 2022. PMID: 35199176 Free PMC article. Review.
-
Severe viral infections and primary immunodeficiencies.Clin Infect Dis. 2011 Nov;53(9):897-909. doi: 10.1093/cid/cir610. Epub 2011 Sep 29. Clin Infect Dis. 2011. PMID: 21960712 Free PMC article. Review.
-
Neuron-intrinsic immunity to viruses in mice and humans.Curr Opin Immunol. 2021 Oct;72:309-317. doi: 10.1016/j.coi.2021.07.004. Epub 2021 Aug 20. Curr Opin Immunol. 2021. PMID: 34425410 Free PMC article. Review.
-
Who and how, DNA sensors in NETs-driven inflammation.Front Immunol. 2023 Apr 28;14:1190177. doi: 10.3389/fimmu.2023.1190177. eCollection 2023. Front Immunol. 2023. PMID: 37187738 Free PMC article. Review.
References
-
- Lemaitre B, et al The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996;86:973–983. - PubMed
-
- Medzhitov R, Preston‐Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997;388:394–397. - PubMed
-
- Poltorak A, et al Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

