Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors
- PMID: 17979880
- PMCID: PMC4401270
- DOI: 10.1111/j.1582-4934.2007.00120.x
Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors
Abstract
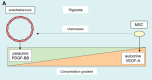
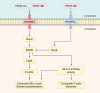
There is now accumulating evidence that bone marrow-derived mesenchymal stem cells (MSCs) make an important contribution to postnatal vasculogenesis, especially during tissue ischaemia and tumour vascularization. Identifying mechanisms which regulate the role of MSCs in vasculogenesis is a key therapeutic objective, since while increased neovascularization can be advantageous during tissue ischaemia, it is deleterious during tumourigenesis. The potent angiogenic stimulant vascular endothelial growth factor (VEGF) is known to regulate MSC mobilization and recruitment to sites of neovascularization, as well as directing the differentiation of MSCs to a vascular cell fate. Despite the fact that MSCs did not express VEGF receptors, we have recently identified that VEGF-A can stimulate platelet-derived growth factor (PDGF) receptors, which regulates MSC migration and proliferation. This review focuses on the role of PDGF receptors in regulating the vascular cell fate of MSCs, with emphasis on the function of the novel VEGF-A/PDGF receptor signalling mechanism.
Figures

References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng Y-J, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into a endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–6. - PubMed
-
- Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–6. - PubMed
-
- Annabi B, Naud E, Lee Y-T, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

