Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6)
- PMID: 17979890
- PMCID: PMC4401281
- DOI: 10.1111/j.1582-4934.2007.00090.x
Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6)
Abstract
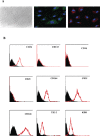
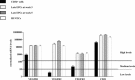
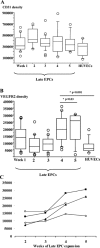
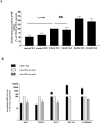
In vitro expansion of late endothelial progenitor cells (EPCs) might yield a cell therapy product useful for myocardial and leg ischaemia, but the influence of EPC expansion on the angiogenic properties of these cells is unknown. In the present study, we investigated the effect of in vitro EPC expansion on vascular endothelial growth factor (VEGF) receptor expression. EPCs were obtained from CD34(+) cord blood cells and expanded for up to 5 weeks. Real-time quantitative reverse-transcription polymerase chain reaction (RT-PCR) showed that VEGFR2 expression, contrary to VEGFR1 and VEGFR3 expression, was significantly higher on expanded EPCs than on freshly isolated CD34(+) cells or on human umbilical vein endothelial cells (HUVECs). Quantitative flow cytometry confirmed that VEGFR2 density on EPCs increased during the expansion process and was significantly higher than on HUVECs. The impact of VEGFR2 increase was studied on the three theoretical steps of angiogenesis, i.e., EPC proliferation, migration and differentiation. VEGFR2 up-regulation had no effect on VEGF-induced cell proliferation, but significantly enhanced EPC migration and pseudotubes formation dependent on integrin alpha(6) subunit overexpression. In vitro expansion of late EPCs increases the expression of VEGFR2, the main VEGF receptor, with possible implications for EPC-based angiogenic therapy.
Figures

Similar articles
-
CD34+ VEGFR-3+ progenitor cells have a potential to differentiate towards lymphatic endothelial cells.J Cell Mol Med. 2014 Mar;18(3):422-33. doi: 10.1111/jcmm.12233. Epub 2014 Jan 22. J Cell Mol Med. 2014. PMID: 24450475 Free PMC article.
-
Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling.Nature. 2012 Mar 18;484(7392):110-4. doi: 10.1038/nature10908. Nature. 2012. PMID: 22426001
-
VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling.Circ Res. 2017 Apr 28;120(9):1414-1425. doi: 10.1161/CIRCRESAHA.116.310477. Epub 2017 Mar 15. Circ Res. 2017. PMID: 28298294 Free PMC article.
-
EPCs and pathological angiogenesis: when good cells go bad.Microvasc Res. 2010 May;79(3):207-16. doi: 10.1016/j.mvr.2010.02.011. Epub 2010 Feb 25. Microvasc Res. 2010. PMID: 20188747 Free PMC article. Review.
-
Angiogenic cell therapy for hepatic fibrosis.Med Mol Morphol. 2006 Mar;39(1):16-21. doi: 10.1007/s00795-006-0311-1. Med Mol Morphol. 2006. PMID: 16575510 Review.
Cited by
-
Interleukin 8 is differently expressed and modulated by PAR-1 activation in early and late endothelial progenitor cells.J Cell Mol Med. 2009 Aug;13(8B):2534-2546. doi: 10.1111/j.1582-4934.2008.00429.x. Epub 2008 Jul 23. J Cell Mol Med. 2009. PMID: 18657231 Free PMC article.
-
Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling.J Neurosci. 2011 May 25;31(21):7682-90. doi: 10.1523/JNEUROSCI.0239-11.2011. J Neurosci. 2011. PMID: 21613481 Free PMC article.
-
The Role of Endothelial Progenitor Cells in Postnatal Vasculogenesis: Implications for Therapeutic Neovascularization and Wound Healing.Adv Wound Care (New Rochelle). 2013 Jul;2(6):283-295. doi: 10.1089/wound.2012.0398. Adv Wound Care (New Rochelle). 2013. PMID: 24527350 Free PMC article. Review.
-
Hypoxia-induced endothelial progenitor cell function is blunted in angiotensinogen knockout mice.Mol Cells. 2014 Jun;37(6):487-96. doi: 10.14348/molcells.2014.0119. Epub 2014 Jun 18. Mol Cells. 2014. PMID: 24938229 Free PMC article.
-
Human Endothelial Colony Forming Cells Express Intracellular CD133 that Modulates their Vasculogenic Properties.Stem Cell Rev Rep. 2019 Aug;15(4):590-600. doi: 10.1007/s12015-019-09881-8. Stem Cell Rev Rep. 2019. PMID: 30879244
References
-
- Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothe-lial cells for angiogenesis. Science. 1997;5302:964–7. - PubMed
-
- Smadja DM, Cornet A, Emmerich J, Aiach M, Gaussem P. Endothelial progenitor cells: Characterization, in vitro expansion, and prospects for autologous cell therapy. Cell Biol Toxicol. 2007;4:223–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

