ADAM8 expression is associated with increased invasiveness and reduced patient survival in pancreatic cancer
- PMID: 17979891
- PMCID: PMC4401277
- DOI: 10.1111/j.1582-4934.2007.00082.x
ADAM8 expression is associated with increased invasiveness and reduced patient survival in pancreatic cancer
Abstract
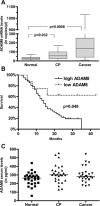
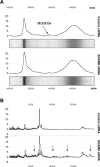
ADAM8 belongs to a family of transmembrane proteins implicated in cell-cell interactions, proteolysis of membrane proteins, and various aspects of carcinogenesis. In the present study, we aimed to evaluate the expression and function of ADAM8 in pancreatic cancer. ADAM8 mRNA levels were analysed by quantitative RT-PCR and correlated to patient survival. Immunohistochemistry was performed to localize ADAM8 in pancreatic tis-sues. Silencing of ADAM8 expression was carried out by transfection with specific siRNA oligonucleotides. Cell growth and invasion assays were used to assess the functional consequences of ADAM8 silencing. SELDI-TOF-MS was performed to detect the proteolytic activity of ADAM8 in pancreatic cancer cells. ADAM8 mRNA was significantly overexpressed in pancreatic ductal adenocarcinoma (PDAC) compared with normal pancreatic tissues (5.3-fold increase; P= 0.0008), and high ADAM8 mRNA and protein expression levels correlated with reduced survival time of PDAC patients (P= 0.048 and P= 0.065, respectively). Silencing of ADAM8 expression did not significantly influence pancreatic cancer cell growth but suppressed invasiveness. In addition, decreased proteolytic activity was measured in cell culture supernatants following silencing of ADAM8. In conclusion, ADAM8 is overexpressed in PDAC, influences cancer cell invasiveness and correlates with reduced survival, suggesting that ADAM8 might be a potential target in pancreatic cancer therapy.
Figures




Similar articles
-
Anti-inflammatory macrophages activate invasion in pancreatic adenocarcinoma by increasing the MMP9 and ADAM8 expression.Med Oncol. 2014 Mar;31(3):884. doi: 10.1007/s12032-014-0884-9. Epub 2014 Feb 14. Med Oncol. 2014. PMID: 24526468
-
The long non-coding RNA NEAT1 contributes to aberrant STAT3 signaling in pancreatic cancer and is regulated by a metalloprotease-disintegrin ADAM8/miR-181a-5p axis.Cell Oncol (Dordr). 2025 Apr;48(2):391-409. doi: 10.1007/s13402-024-01001-0. Epub 2024 Oct 16. Cell Oncol (Dordr). 2025. PMID: 39412616 Free PMC article.
-
ADAM8 as a drug target in pancreatic cancer.Nat Commun. 2015 Jan 28;6:6175. doi: 10.1038/ncomms7175. Nat Commun. 2015. PMID: 25629724 Free PMC article.
-
ADAM8 in invasive cancers: links to tumor progression, metastasis, and chemoresistance.Clin Sci (Lond). 2019 Jan 11;133(1):83-99. doi: 10.1042/CS20180906. Print 2019 Jan 15. Clin Sci (Lond). 2019. PMID: 30635388 Review.
-
A jack of all trades - ADAM8 as a signaling hub in inflammation and cancer.FEBS J. 2024 Sep;291(18):3989-4008. doi: 10.1111/febs.17034. Epub 2023 Dec 22. FEBS J. 2024. PMID: 38097912 Review.
Cited by
-
A disintegrin and metalloproteinase 8 induced epithelial-mesenchymal transition to promote the invasion of colon cancer cells via TGF-β/Smad2/3 signalling pathway.J Cell Mol Med. 2020 Nov;24(22):13058-13069. doi: 10.1111/jcmm.15907. Epub 2020 Sep 20. J Cell Mol Med. 2020. PMID: 32954649 Free PMC article.
-
The versatile roles of ADAM8 in cancer cell migration, mechanics, and extracellular matrix remodeling.Front Cell Dev Biol. 2023 Feb 23;11:1130823. doi: 10.3389/fcell.2023.1130823. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910158 Free PMC article. Review.
-
ADAM8-Dependent Extracellular Signaling in the Tumor Microenvironment Involves Regulated Release of Lipocalin 2 and MMP-9.Int J Mol Sci. 2022 Feb 10;23(4):1976. doi: 10.3390/ijms23041976. Int J Mol Sci. 2022. PMID: 35216088 Free PMC article.
-
The Metalloprotease-Disintegrin ADAM8 Alters the Tumor Suppressor miR-181a-5p Expression Profile in Glioblastoma Thereby Contributing to Its Aggressiveness.Front Oncol. 2022 Mar 15;12:826273. doi: 10.3389/fonc.2022.826273. eCollection 2022. Front Oncol. 2022. PMID: 35371977 Free PMC article.
-
ADAM8 expression in invasive breast cancer promotes tumor dissemination and metastasis.EMBO Mol Med. 2014 Feb;6(2):278-94. doi: 10.1002/emmm.201303373. Epub 2013 Dec 27. EMBO Mol Med. 2014. PMID: 24375628 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. - PubMed
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. - PubMed
-
- Kleeff J, Michalski C, Friess H, Buchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111–8. - PubMed
-
- Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW. Ectodomain shedding of the neural recognition molecule CHL1 by the metal-loprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem. 2004;279:16083–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

