Agonist treatment did not affect association of mu opioid receptors with lipid rafts and cholesterol reduction had opposite effects on the receptor-mediated signaling in rat brain and CHO cells
- PMID: 17980352
- PMCID: PMC3715317
- DOI: 10.1016/j.brainres.2007.09.096
Agonist treatment did not affect association of mu opioid receptors with lipid rafts and cholesterol reduction had opposite effects on the receptor-mediated signaling in rat brain and CHO cells
Abstract
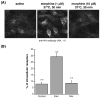
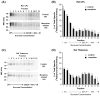
Lipid rafts are small cholesterol- and glycosphingolipid-enriched membrane subdomains. Here we compared the mu opioid receptor (MOR)-lipid rafts relationship in the rat brain, where neurons have non-caveolae rafts, and in CHO cells stably transfected with HA-rat MOR (CHO-HA-rMOR), which are enriched in caveolae. Membranes of rat caudate putamen (CPu) and thalamus or CHO-HA-rMOR cells were homogenized, sonicated in a detergent-free 0.5 M Na(2)CO(3) buffer and fractionated through sucrose density gradients. Western blot and [(3)H]diprenorphine binding showed that approximately 70% of MOR in CHO-HA-rMOR was present in low-density (5-20% sucrose) fractions enriched in cholesterol and/or ganglioside M1 (GM1) (lipid rafts) in plasma membranes, whereas about 70% and 45% of MOR in CPu and thalamus, respectively, were associated with lipid rafts. Incubation with a saturating concentration of etorphine or morphine at 37 degrees C for 30 min failed to change the MOR location in rafts in CHO-HA-rMOR, indicating that the internalized MOR does not move out of rafts, in contrast to the delta opioid receptor. In vivo, rafts association of MOR in CPu and thalamus was not affected significantly in rats implanted with two 75-mg morphine pellets for 72 h. In addition, cholesterol reduction by methyl-beta-cyclodextrin (MCD) disrupted rafts and shifted MOR to higher density fractions in both CHO-HA-rMOR and CPu membranes. However, MCD treatment had opposite impacts on MOR signaling in the two tissues: it attenuated MOR-mediated [(35)S]GTPgammaS binding in CPu but enhanced it in CHO-HA-rMOR.
Figures




Similar articles
-
Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells.Biochem Pharmacol. 2007 Feb 15;73(4):534-49. doi: 10.1016/j.bcp.2006.10.032. Epub 2006 Nov 3. Biochem Pharmacol. 2007. PMID: 17141202 Free PMC article.
-
Brain region-specific N-glycosylation and lipid rafts association of the rat mu opioid receptor.Biochem Biophys Res Commun. 2008 Jan 4;365(1):82-8. doi: 10.1016/j.bbrc.2007.10.128. Epub 2007 Oct 31. Biochem Biophys Res Commun. 2008. PMID: 17980152 Free PMC article.
-
Localization of the kappa opioid receptor in lipid rafts.J Pharmacol Exp Ther. 2006 Jun;317(3):1295-306. doi: 10.1124/jpet.105.099507. Epub 2006 Feb 27. J Pharmacol Exp Ther. 2006. PMID: 16505160
-
Lipid rafts, cholesterol, and the brain.Neuropharmacology. 2008 Dec;55(8):1265-73. doi: 10.1016/j.neuropharm.2008.02.019. Epub 2008 Mar 14. Neuropharmacology. 2008. PMID: 18402986 Free PMC article. Review.
-
Role of Lipid Rafts in Pathogen-Host Interaction - A Mini Review.Front Immunol. 2022 Jan 20;12:815020. doi: 10.3389/fimmu.2021.815020. eCollection 2021. Front Immunol. 2022. PMID: 35126371 Free PMC article. Review.
Cited by
-
Dynamic lateral organization of opioid receptors (kappa, muwt and muN40D ) in the plasma membrane at the nanoscale level.Traffic. 2018 May 28;19(9):690-709. doi: 10.1111/tra.12582. Online ahead of print. Traffic. 2018. PMID: 29808515 Free PMC article.
-
Interaction of drugs with lipid raft membrane domains as a possible target.Drug Target Insights. 2020 Dec 22;14:34-47. doi: 10.33393/dti.2020.2185. eCollection 2020. Drug Target Insights. 2020. PMID: 33510571 Free PMC article. Review.
-
Sex difference in κ-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5'-O-(3-[35S]thiotriphosphate) binding in the guinea pig.J Pharmacol Exp Ther. 2011 Nov;339(2):438-50. doi: 10.1124/jpet.111.183905. Epub 2011 Aug 12. J Pharmacol Exp Ther. 2011. PMID: 21841040 Free PMC article.
-
Detection of the endogenous mu opioid receptor (mopr) in brain.Front Biosci (Elite Ed). 2009 Jun 1;1(1):220-7. doi: 10.2741/E21. Front Biosci (Elite Ed). 2009. PMID: 19482639 Free PMC article. Review.
-
Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase.J Biol Chem. 2009 Aug 14;284(33):22108-22122. doi: 10.1074/jbc.M109.030411. Epub 2009 Jun 11. J Biol Chem. 2009. PMID: 19520863 Free PMC article.
References
-
- Bell J, Adler MW. Comparison of peripheral and central administration of naloxone in precipitating abstinence in morphine-dependent rats. Drug Alcohol Depend. 1988;21:189–194. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
-
- Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

