Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy
- PMID: 17981266
- PMCID: PMC2352150
- DOI: 10.1016/j.biopsych.2007.09.019
Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy
Abstract
Background: Brain-derived neurotrophic factor (BDNF) plays an important role in neural plasticity in the adult nervous system and has been suggested as a target gene for antidepressant treatment. The neurotrophic hypothesis of depression suggests that loss of BDNF from the hippocampus contributes to an increased vulnerability for depression, whereas upregulation of BDNF in the hippocampus is suggested to mediate antidepressant efficacy.
Methods: We have used a viral-mediated gene transfer approach to assess the role of BDNF in subregions of the hippocampus in a broad array of behavioral paradigms, including depression-like behavior and antidepressant responses. We have combined the adeno-associated virus (AAV) with the Cre/loxP site-specific recombination system to induce the knockout of BDNF selectively in either the CA1 or dentate gyrus (DG) subregions of the hippocampus.
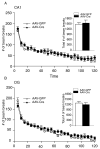
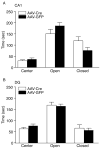
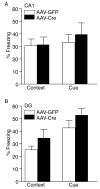
Results: We show that the loss of BDNF in either the CA1 or the DG of the hippocampus does not alter locomotor activity, anxiety-like behavior, fear conditioning, or depression-related behaviors. However, the selective loss of BDNF in the DG but not the CA1 region attenuates the actions of desipramine and citalopram in the forced swim test.
Conclusions: These data suggest that the loss of hippocampal BDNF per se is not sufficient to mediate depression-like behavior. However, these results support the view that BDNF in the DG might be essential in mediating the therapeutic effect of antidepressants.
Figures


Comment in
-
The when and where of BDNF and the antidepressant response.Biol Psychiatry. 2008 Apr 1;63(7):640-1. doi: 10.1016/j.biopsych.2008.01.008. Biol Psychiatry. 2008. PMID: 18329441 Review. No abstract available.
References
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. - PubMed
-
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. - PubMed
-
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. - PubMed
-
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. - PubMed
-
- Sanders AR, Detera-Wadleigh SD, Gershon ES. Molecular genetics of mood disorders. In: Nestler EJ, Charney DS, Bunney BS, editors. Neurobiology of Mental Illness. New York: Oxford; 1999. pp. 299–316.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

