Role of N-terminal domain and accessory subunits in controlling deactivation-inactivation coupling of Kv4.2 channels
- PMID: 17981906
- PMCID: PMC2212696
- DOI: 10.1529/biophysj.107.111344
Role of N-terminal domain and accessory subunits in controlling deactivation-inactivation coupling of Kv4.2 channels
Abstract
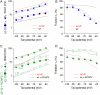
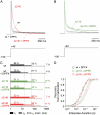
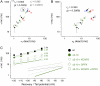
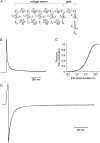
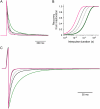
We examined the relationship between deactivation and inactivation in Kv4.2 channels. In particular, we were interested in the role of a Kv4.2 N-terminal domain and accessory subunits in controlling macroscopic gating kinetics and asked if the effects of N-terminal deletion and accessory subunit coexpression conform to a kinetic coupling of deactivation and inactivation. We expressed Kv4.2 wild-type channels and N-terminal deletion mutants in the absence and presence of Kv channel interacting proteins (KChIPs) and dipeptidyl aminopeptidase-like proteins (DPPs) in human embryonic kidney 293 cells. Kv4.2-mediated A-type currents at positive and deactivation tail currents at negative membrane potentials were recorded under whole-cell voltage-clamp and analyzed by multi-exponential fitting. The observed changes in Kv4.2 macroscopic inactivation kinetics caused by N-terminal deletion, accessory subunit coexpression, or a combination of the two maneuvers were compared with respective changes in deactivation kinetics. Extensive correlation analyses indicated that modulatory effects on deactivation closely parallel respective effects on inactivation, including both onset and recovery kinetics. Searching for the structural determinants, which control deactivation and inactivation, we found that in a Kv4.2 Delta 2-10 N-terminal deletion mutant both the initial rapid phase of macroscopic inactivation and tail current deactivation were slowed. On the other hand, the intermediate and slow phase of A-type current decay, recovery from inactivation, and tail current decay kinetics were accelerated in Kv4.2 Delta 2-10 by KChIP2 and DPPX. Thus, a Kv4.2 N-terminal domain, which may control both inactivation and deactivation, is not necessary for active modulation of current kinetics by accessory subunits. Our results further suggest distinct mechanisms for Kv4.2 gating modulation by KChIPs and DPPs.
Figures

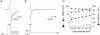





References
-
- Baldwin, T. J., M. L. Tsaur, G. A. Lopez, Y. N. Jan, and L. Y. Jan. 1991. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 7:471–483. - PubMed
-
- Dixon, J. E., W. Shi, H. S. Wang, C. McDonald, H. Yu, R. S. Wymore, I. S. Cohen, and D. McKinnon. 1996. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ. Res. 79:659–668. - PubMed
-
- Serodio, P., C. Kentros, and B. Rudy. 1994. Identification of molecular components of A-type channels activating at subthreshold potentials. J. Neurophysiol. 72:1516–1529. - PubMed
-
- Sheng, M., Y. J. Liao, Y. N. Jan, and L. Y. Jan. 1993. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 365:72–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

