The amino acid valine is secreted in continuous-flow bacterial biofilms
- PMID: 17981982
- PMCID: PMC2223729
- DOI: 10.1128/JB.01405-07
The amino acid valine is secreted in continuous-flow bacterial biofilms
Abstract
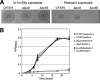
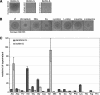
Biofilms are structured communities characterized by distinctive gene expression patterns and profound physiological changes compared to those of planktonic cultures. Here, we show that many gram-negative bacterial biofilms secrete high levels of a small-molecular-weight compound, which inhibits the growth of only Escherichia coli K-12 and a rare few other natural isolates. We demonstrate both genetically and biochemically that this molecule is the amino acid valine, and we provide evidence that valine production within biofilms results from metabolic changes occurring within high-density biofilm communities when carbon sources are not limiting. This finding identifies a natural environment in which bacteria can encounter high amounts of valine, and we propose that in-biofilm valine secretion may be the long-sought reason for widespread but unexplained valine resistance found in most enterobacteria. Our results experimentally validate the postulated production of metabolites that is characteristic of the conditions associated with some biofilm environments. The identification of such molecules may lead to new approaches for biofilm monitoring and control.
Figures





Similar articles
-
Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms.Antimicrob Agents Chemother. 2007 Oct;51(10):3650-8. doi: 10.1128/AAC.00601-07. Epub 2007 Jul 30. Antimicrob Agents Chemother. 2007. PMID: 17664315 Free PMC article.
-
Enterohemorrhagic Escherichia coli biofilms are inhibited by 7-hydroxyindole and stimulated by isatin.Appl Environ Microbiol. 2007 Jul;73(13):4100-9. doi: 10.1128/AEM.00360-07. Epub 2007 May 4. Appl Environ Microbiol. 2007. PMID: 17483266 Free PMC article.
-
Transcription in Escherichia coli PHL628 biofilms.FEMS Microbiol Lett. 2007 Mar;268(2):237-43. doi: 10.1111/j.1574-6968.2006.00585.x. Epub 2007 Jan 12. FEMS Microbiol Lett. 2007. PMID: 17227468
-
Biofilm-specific antibiotic resistance.Future Microbiol. 2012 Sep;7(9):1061-72. doi: 10.2217/fmb.12.76. Future Microbiol. 2012. PMID: 22953707 Review.
-
Bacterial resistance in biofilm-associated bacteria.Future Microbiol. 2015;10(11):1743-50. doi: 10.2217/fmb.15.69. Epub 2015 Oct 30. Future Microbiol. 2015. PMID: 26517598 Review.
Cited by
-
Gas chromatography-mass spectrometry-based metabolite profiling of Salmonella enterica serovar Typhimurium differentiates between biofilm and planktonic phenotypes.Appl Environ Microbiol. 2015 Apr;81(8):2660-6. doi: 10.1128/AEM.03658-14. Epub 2015 Jan 30. Appl Environ Microbiol. 2015. PMID: 25636852 Free PMC article.
-
Cellular export of sugars and amino acids: role in feeding other cells and organisms.Plant Physiol. 2021 Dec 4;187(4):1893-1914. doi: 10.1093/plphys/kiab228. Plant Physiol. 2021. PMID: 34015139 Free PMC article. Review.
-
Dynamic Metabolic Response to (p)ppGpp Accumulation in Pseudomonas putida.Front Microbiol. 2022 Apr 14;13:872749. doi: 10.3389/fmicb.2022.872749. eCollection 2022. Front Microbiol. 2022. PMID: 35495732 Free PMC article.
-
The ribonuclease PNPase is a key regulator of biofilm formation in Listeria monocytogenes and affects invasion of host cells.NPJ Biofilms Microbiomes. 2023 Jun 7;9(1):34. doi: 10.1038/s41522-023-00397-1. NPJ Biofilms Microbiomes. 2023. PMID: 37286543 Free PMC article.
-
Insights Into the Dynamics and Composition of Biofilm Formed by Environmental Isolate of Enterobacter cloacae.Front Microbiol. 2022 Jul 5;13:877060. doi: 10.3389/fmicb.2022.877060. eCollection 2022. Front Microbiol. 2022. PMID: 35865928 Free PMC article.
References
-
- Andersen, D. C., J. Swartz, T. Ryll, N. Lin, and B. Snedecor. 2001. Metabolic oscillations in an E. coli fermentation. Biotechnol. Bioeng. 75212-218. - PubMed
-
- Balzer, G. J., and R. J. McLean. 2002. The stringent response genes relA and spoT are important for Escherichia coil biofilms under slow-growth conditions. Can. J. Microbiol. 48675-680. - PubMed
-
- Beloin, C., and J. M. Ghigo. 2005. Finding gene-expression patterns in bacterial biofilms. Trends Microbiol. 1316-19. - PubMed
-
- Beloin, C., J. Valle, P. Latour-Lambert, P. Faure, M. Kzreminski, D. Balestrino, J. A. Haagensen, S. Molin, G. Prensier, B. Arbeille, and J. M. Ghigo. 2004. Global impact of mature biofilm lifestyle on Escherichia coli K-12 gene expression. Mol. Microbiol. 51659-674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

