Glufosinate ammonium-induced pathogen inhibition and defense responses culminate in disease protection in bar-transgenic rice
- PMID: 17981989
- PMCID: PMC2230565
- DOI: 10.1104/pp.107.105890
Glufosinate ammonium-induced pathogen inhibition and defense responses culminate in disease protection in bar-transgenic rice
Abstract
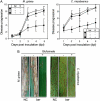
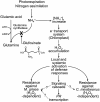
Glufosinate ammonium diminished developments of rice (Oryza sativa) blast and brown leaf spot in 35S:bar-transgenic rice. Pre- and postinoculation treatments of this herbicide reduced disease development. Glufosinate ammonium specifically impeded appressorium formation of the pathogens Magnaporthe grisea and Cochliobolus miyabeanus on hydrophobic surface and on transgenic rice. In contrast, conidial germination remained unaffected. Glufosinate ammonium diminished mycelial growth of two pathogens; however, this inhibitory effect was attenuated in malnutrition conditions. Glufosinate ammonium caused slight chlorosis and diminished chlorophyll content; however, these alterations were almost completely restored in transgenic rice within 7 d. Glufosinate ammonium triggered transcriptions of PATHOGENESIS-RELATED (PR) genes and hydrogen peroxide accumulation in transgenic rice and PR1 transcription in Arabidopsis (Arabidopsis thaliana) wild-type ecotype Columbia harboring 35S:bar construct. All transgenic Arabidopsis showed robust hydrogen peroxide accumulation by glufosinate ammonium. This herbicide also induced PR1 transcription in etr1 and jar1 expressing bar; however, no expression was observed in NahG and npr1. Fungal infection did not alter transcriptions of PR genes and hydrogen peroxide accumulation induced by glufosinate ammonium. Infiltration of glufosinate ammonium did not affect appressorium formation of M. grisea in vivo but inhibited blast disease development. Hydrogen peroxide scavengers nullified blast protection and transcriptions of PR genes by glufosinate ammonium; however, they did not affect brown leaf spot progression. In sum, both direct inhibition of pathogen infection and activation of defense systems were responsible for disease protection in bar-transgenic rice.
Figures







References
-
- Agrawal GK, Jwa NS, Iwahashi H, Rakwal R (2003) Importance of ascorbate peroxidases OsAPX1 and OsAPX2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Gene 322 93–103 - PubMed
-
- Ahn IP, Kim S, Kang S, Suh SC, Lee YH (2005. a) Rice defense mechanisms against Cochliobolus miyabeanus and Magnaporthe grisea are distinct. Phytopathology 95 1248–1255 - PubMed
-
- Ahn IP, Lee SW, Suh SC (2007. b) Rhizobacteria-induced priming in Arabidopsis is dependent on ethylene, jasmonic acid and NPR1. Mol Plant Microbe Interact 20 759–768 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

