Feedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cells
- PMID: 17982172
- PMCID: PMC2190722
- DOI: 10.1093/nar/gkm933
Feedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cells
Abstract
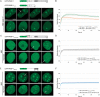
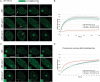
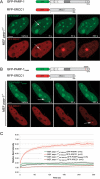
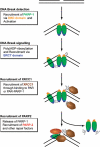
Genome integrity is constantly threatened by DNA lesions arising from numerous exogenous and endogenous sources. Survival depends on immediate recognition of these lesions and rapid recruitment of repair factors. Using laser microirradiation and live cell microscopy we found that the DNA-damage dependent poly(ADP-ribose) polymerases (PARP) PARP-1 and PARP-2 are recruited to DNA damage sites, however, with different kinetics and roles. With specific PARP inhibitors and mutations, we could show that the initial recruitment of PARP-1 is mediated by the DNA-binding domain. PARP-1 activation and localized poly(ADP-ribose) synthesis then generates binding sites for a second wave of PARP-1 recruitment and for the rapid accumulation of the loading platform XRCC1 at repair sites. Further PARP-1 poly(ADP-ribosyl)ation eventually initiates the release of PARP-1. We conclude that feedback regulated recruitment of PARP-1 and concomitant local poly(ADP-ribosyl)ation at DNA lesions amplifies a signal for rapid recruitment of repair factors enabling efficient restoration of genome integrity.
Figures




References
-
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. - PubMed
-
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. - PubMed
-
- Mackey ZB, Niedergang C, Murcia JM, Leppard J, Au K, Chen J, de Murcia G, Tomkinson AE. DNA ligase III is recruited to DNA strand breaks by a zinc finger motif homologous to that of poly(ADP-ribose) polymerase. Identification of two functionally distinct DNA binding regions within DNA ligase III. J. Biol. Chem. 1999;274:21679–21687. - PubMed
-
- Pleschke JM, Kleczkowska HE, Strohm M, Althaus FR. Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 2000;275:40974–40980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

