Common activation of canonical Wnt signaling in pancreatic adenocarcinoma
- PMID: 17982507
- PMCID: PMC2048934
- DOI: 10.1371/journal.pone.0001155
Common activation of canonical Wnt signaling in pancreatic adenocarcinoma
Abstract
Pancreatic ductal adenocarcinoma (PDA) is an extremely aggressive malignancy, which carries a dismal prognosis. Activating mutations of the Kras gene are common to the vast majority of human PDA. In addition, recent studies have demonstrated that embryonic signaling pathway such as Hedgehog and Notch are inappropriately upregulated in this disease. The role of another embryonic signaling pathway, namely the canonical Wnt cascade, is still controversial. Here, we use gene array analysis as a platform to demonstrate general activation of the canonical arm of the Wnt pathway in human PDA. Furthermore, we provide evidence for Wnt activation in mouse models of pancreatic cancer. Our results also indicate that Wnt signaling might be activated downstream of Hedgehog signaling, which is an early event in PDA evolution. Wnt inhibition blocked proliferation and induced apoptosis of cultured adenocarcinoma cells, thereby providing evidence to support the development of novel therapeutical strategies for Wnt inhibition in pancreatic adenocarcinoma.
Conflict of interest statement
Figures
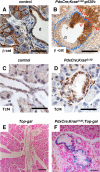
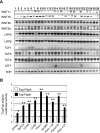
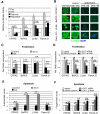

References
-
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. - PubMed
-
- Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. - PubMed
-
- Biankin AV, Kench JG, Dijkman FP, Biankin SA, Henshall SM. Molecular pathogenesis of precursor lesions of pancreatic ductal adenocarcinoma. Pathology. 2003;35:14–24. - PubMed
-
- Biankin AV, Kench JG, Morey AL, Lee CS, Biankin SA, et al. Overexpression of p21(WAF1/CIP1) is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res. 2001;61:8830–8837. - PubMed
-
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

