Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis
- PMID: 17983266
- PMCID: PMC2048529
- DOI: 10.1371/journal.ppat.0030161
Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis
Abstract
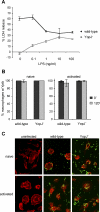
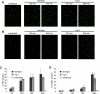
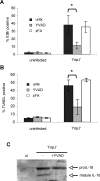
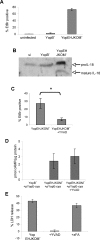
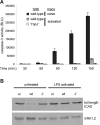
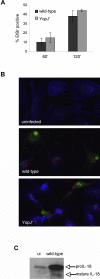
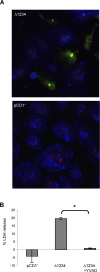
Infection of macrophages by Yersinia species results in YopJ-dependent apoptosis, and naïve macrophages are highly susceptible to this form of cell death. Previous studies have demonstrated that macrophages activated with lipopolysaccharide (LPS) prior to infection are resistant to YopJ-dependent cell death; we found this simultaneously renders macrophages susceptible to killing by YopJ(-) Yersinia pseudotuberculosis (Yptb). YopJ(-) Yptb-induced macrophage death was dependent on caspase-1 activation, resulting in rapid permeability to small molecules, followed by membrane breakdown and DNA damage, and accompanied by cleavage and release of proinflammatory interleukin-18. Induction of caspase-1-dependent death, or pyroptosis, required the bacterial type III translocon but none of its known translocated proteins. Wild-type Yptb infection also triggered pyroptosis: YopJ-dependent activation of proapoptotic caspase-3 was significantly delayed in activated macrophages and resulted in caspase-1-dependent pyroptosis. The transition to susceptibility was not limited to LPS activation; it was also seen in macrophages activated with other Toll-like receptor (TLR) ligands and intact nonviable bacteria. Yptb infection triggered macrophage activation and activation of caspase-1 in vivo. Y. pestis infection of activated macrophages also stimulated caspase-1 activation. These results indicate that host signaling triggered by TLR and other activating ligands during the course of Yersinia infection redirects both the mechanism of host cell death and the downstream consequences of death by shifting from noninflammatory apoptosis to inflammatory pyroptosis.
Conflict of interest statement
Figures

References
-
- Zhang Y, Bliska JB. Immunol Role of macrophage apoptosis in the pathogenesis of Yersinia. Curr Top Microbiol. 2005;289:151–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

