Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells
- PMID: 17984096
- PMCID: PMC2729092
- DOI: 10.1074/jbc.M708250200
Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells
Abstract
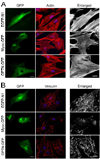
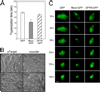
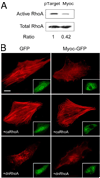
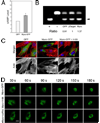
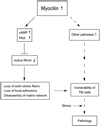
Myocilin is a gene linked to the most common form of glaucoma, a major blinding disease. The trabecular meshwork (TM), a specialized eye tissue, is believed to be involved, at least in part, in the development of glaucoma. The myocilin expression is known to be up-regulated by glucocorticoids in TM cells, and an altered myocilin level may be the culprit in conditions such as corticosteroid glaucoma. Wild type myocilin, when transfected into cultured human TM cells, induced a dramatic loss of actin stress fibers and focal adhesions. Myocilin transfectants displayed a heightened sensitivity to trypsin. Adhesion to fibronectin, collagens, and vitronectin was compromised. The fibronectin deposition and the levels of fibronectin protein and mRNA were also reduced in myocilin transfectants. The fibronectin deposition could be restored by treatment with lysophosphatidic acid, a Rho stimulator. Assays further revealed that upon myocilin overexpression, the activity of RhoA was diminished, whereas the cAMP level and the protein kinase A (PKA) activity were augmented. Myocilin protein did not affect actin polymerization. The collapse of actin stress fibers and increased trypsin sensitivity from myocilin transfection could be reverted by co-expression of constitutively active RhoA or by treatment with PKA inhibitor H-89. The PKA activity, however, was not modified by co-expression of either constitutively active or dominant negative RhoA. These results demonstrate that myocilin has a de-adhesive activity and triggers signaling events. cAMP/PKA activation and the downstream Rho inhibition are possible mechanisms by which myocilin in overabundance may lead to TM cell or tissue damage.
Figures





References
-
- Bill A. Invest. Ophthalmol. Vis. Sci. 1975;14:1–3. - PubMed
-
- Yue BYJT. Surv. Ophthalmol. 1996;40:379–390. - PubMed
-
- Zhou L, Fukuchi T, Kawa JE, Higginbotham EJ, Yue BYJT. Invest. Ophthalmol. Vis. Res. 1995;36:787–795. - PubMed
-
- Calthorpe CM, Grierson I. Exp. Eye Res. 1990;51:39–48. - PubMed
-
- Stumpff F, Wiederholt M. Ophthalmologica. 2000;214:33–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

