Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2
- PMID: 17984209
- PMCID: PMC2223649
- DOI: 10.1128/IAI.01199-07
Enteropathogenic Escherichia coli O125:H6 triggers attaching and effacing lesions on human intestinal biopsy specimens independently of Nck and TccP/TccP2
Abstract
Typical enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) employ either Nck, TccP/TccP2, or Nck and TccP/TccP2 pathways to activate the neuronal Wiskott-Aldrich syndrome protein (N-WASP) and to trigger actin polymerization in cultured cells. This phenotype is used as a marker for the pathogenic potential of EPEC and EHEC strains. In this paper we report that EPEC O125:H6, which represents a large category of strains, lacks the ability to utilize either Nck or TccP/TccP2 and hence triggers actin polymerization in vitro only inefficiently. However, we show that infection of human intestinal biopsies with EPEC O125:H6 results in formation of typical attaching and effacing lesions. Expression of TccP in EPEC O125:H6, which harbors an EHEC O157-like Tir, resulted in efficient actin polymerization in vitro and enhanced colonization of human intestinal in vitro organ cultures with detectable N-WASP and electron-dense material at the site of bacterial adhesion. These results show the existence of a natural category of EPEC that colonizes the gut mucosa using Nck- and TccP-independent mechanisms. Importantly, the results highlight yet again the fact that conclusions made on the basis of in vitro cell culture models cannot be extrapolated wholesale to infection of mucosal surfaces and that the ability to induce actin polymerization on cultured cells should not be used as a definitive marker for EPEC and EHEC virulence.
Figures
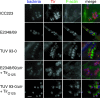



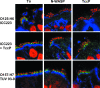

References
-
- Campellone, K. G., A. Giese, D. J. Tipper, and J. M. Leong. 2002. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol. Microbiol. 431227-1241. - PubMed
-
- Campellone, K. G., and J. M. Leong. 2005. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol. Microbiol. 56416-432. - PubMed
-
- Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7217-228. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

