Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis
- PMID: 17984302
- PMCID: PMC2118521
- DOI: 10.1084/jem.20062299
Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis
Abstract
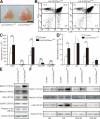
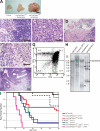
Cell proliferation is strictly controlled during differentiation. In T cell development, the cell cycle is normally arrested at the CD4(+)CD8(+) stage, but the mechanism underlying such differentiation-specific exit from the cell cycle has been unclear. Fbxw7 (also known as Fbw7, Sel-10, hCdc4, or hAgo), an F-box protein subunit of an SCF-type ubiquitin ligase complex, induces the degradation of positive regulators of the cell cycle, such as c-Myc, c-Jun, cyclin E, and Notch. FBXW7 is often mutated in a subset of human cancers. We have now achieved conditional inactivation of Fbxw7 in the T cell lineage of mice and found that the cell cycle is not arrested at the CD4(+)CD8(+) stage in the homozygous mutant animals. The mutant mice manifested thymic hyperplasia as a result of c-Myc accumulation and eventually developed thymic lymphoma. In contrast, mature T cells of the mutant mice failed to proliferate in response to mitogenic stimulation and underwent apoptosis in association with accumulation of c-Myc and p53. These latter abnormalities were corrected by deletion of p53. Our results suggest that Fbxw7 regulates the cell cycle in a differentiation-dependent manner, with its loss resulting in c-Myc accumulation that leads to hyperproliferation in immature T cells but to p53-dependent cell-cycle arrest and apoptosis in mature T cells.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

