Inheritance of cortical ER in yeast is required for normal septin organization
- PMID: 17984322
- PMCID: PMC2064793
- DOI: 10.1083/jcb.200708205
Inheritance of cortical ER in yeast is required for normal septin organization
Abstract
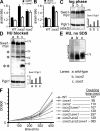
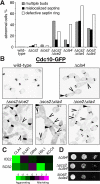
How cells monitor the distribution of organelles is largely unknown. In budding yeast, the largest subdomain of the endoplasmic reticulum (ER) is a network of cortical ER (cER) that adheres to the plasma membrane. Delivery of cER from mother cells to buds, which is termed cER inheritance, occurs as an orderly process early in budding. We find that cER inheritance is defective in cells lacking Scs2, a yeast homologue of the integral ER membrane protein VAP (vesicle-associated membrane protein-associated protein) conserved in all eukaryotes. Scs2 and human VAP both target yeast bud tips, suggesting a conserved action of VAP in attaching ER to sites of polarized growth. In addition, the loss of either Scs2 or Ice2 (another protein involved in cER inheritance) perturbs septin assembly at the bud neck. This perturbation leads to a delay in the transition through G2, activating the Saccharomyces wee1 kinase (Swe1) and the morphogenesis checkpoint. Thus, we identify a mechanism involved in sensing the distribution of ER.
Figures
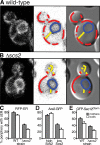


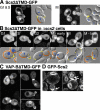
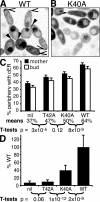

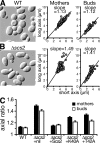
References
-
- Ayscough, K.R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D.G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416. - PMC - PubMed
-
- Bardo, S., M.G. Cavazzini, and N. Emptage. 2006. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol. Sci. 27:78–84. - PubMed
-
- Beilharz, T., B. Egan, P.A. Silver, K. Hofmann, and T. Lithgow. 2003. Bipartite signals mediate subcellular targeting of tail-anchored membrane proteins in Saccharomyces cerevisiae. J. Biol. Chem. 278:8219–8223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

