Effects of an enhanced secondary prevention program for patients with heart disease: a prospective randomized trial
- PMID: 17985009
- PMCID: PMC2651931
- DOI: 10.1016/s0828-282x(07)70875-9
Effects of an enhanced secondary prevention program for patients with heart disease: a prospective randomized trial
Abstract
Background: Secondary prevention medications in cardiac patients improve outcomes. However, prescription rates for these drugs and long-term adherence are suboptimal.
Objective: To determine whether an enhanced secondary prevention program improves outcomes.
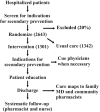
Methods: Hospitalized patients with indications for secondary prevention medications were randomly assigned to either usual care or an intervention arm, in which an intensive program was used to optimize prescription rates and long-term adherence. Follow-up was 19 months.
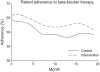
Results: A total of 2643 patients were randomly assigned in the study; 1342 patients were assigned to usual care and 1301 patients were assigned to the intervention arm. Prescription rates were near optimal except for lipid-lowering medications. Rehospitalization rates per 100 patients were 136.2 and 132.6 over 19 months in the usual care and intervention groups, respectively (P=0.59). Total days in hospital per patient were similar (10.9 days in the usual care group versus 10.2 days in the intervention group; P not significant). Crude mortality was 6.2% and 5.5% in the usual care and intervention groups, respectively, with no significant difference (P=0.15) in overall survival. Post hoc analysis suggested that after the study team became experienced, days in hospital per patient were reduced by the program (11.1+/-0.91 and 8.9+/-0.61 in the usual care and intervention groups, respectively; P<0.05).
Conclusions: The intervention program failed to improve outcomes in the present study. One explanation for these results is the near optimal physician compliance with guidelines in both groups. It is also possible that a substantial learning curve for the staff was involved, as suggested by the reduction in total days in hospital in the intervention patients during the second part of the study.
HISTORIQUE :: Les médicaments de prévention secondaire améliorent les issues chez les patients cardiaques. Cependant, les taux de prescription de ces médicaments et l’observance à long terme sont sous-optimaux.
OBJECTIF :: Déterminer si un programme de prévention secondaire amélioré assure de meilleures issues.
MÉTHODOLOGIE :: Des patients hospitalisés ayant des indications de prendre de médicaments de prévention secondaire ont été divisés au hasard entre les soins habituels ou une intervention sous forme de programme intensif pour optimiser les taux de prescription et l’observance à long terme. Le suivi a duré 19 mois.
RÉSULTATS :: Au total, 2 643 patients ont été sélectionnés au hasard dans l’étude : 1 342 patients ont été attribués au groupe de soins habituels, et 1 301 patients, au groupe d’intervention. Les taux de prescription étaient presque optimaux, sauf pour les médicaments visant à réduire les taux de lipide. Les taux de réhospitalisation par tranche de 100 patients étaient de 136,2 et 132, 6 sur 19 mois au sein des groupes de soins habituels et d’intervention, respectivement (p = 0,59). Les jours totaux d’hospitalisation par patient étaient similaires (10,9 jours au sein du groupe de soins habituels et 10,2 au sein du groupe d’intervention; p non significatif). Le taux brut de mortalité était de 6,2 % et 5,5 % au sein des groupes de soins habituels et d’intervention, respectivement, sans différence significative (p = 0,15) de survie globale. L’analyse ultérieure laisse supposer que lorsque l’équipe de l’étude a pris de l’expérience, les jours d’hospitalisation par patient ont diminué (11,1±0,91 et 8,9±0,61 dans les groupes de soins habituels et d’intervention, respectivement; p < 0,05).
CONCLUSIONS :: Le programme d’intervention n’améliorait pas les issues dans la présente étude. L’une des explications de ces résultats, c’est l’observance presque optimale des lignes directrices au sein des deux groupes. Il est également possible qu’une courbe d’apprentissage importante du personnel soit entrée en ligne de compte, telle que le laisse supposer la réduction des jours totaux d’hospitalisation chez les patients du groupe d’intervention pendant la deuxième partie de l’étude.
Figures





References
-
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. - PubMed
-
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. - PubMed
-
- Genest J, Pedersen TR. Prevention of cardiovascular ischemic events: High-risk and secondary prevention. Circulation. 2003;107:2059–65. - PubMed
-
- van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: An individual patient meta-analysis. JAMA. 2002;288:2441–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

