Total synthesis of (-)-pseudolaric acid B
- PMID: 17985906
- PMCID: PMC2535803
- DOI: 10.1021/ja076165q
Total synthesis of (-)-pseudolaric acid B
Abstract
We report the enantioselective synthesis of pseudolaric acid B (1a), a diterpene acid isolated from the bark of Pseudolarix kaempferi Gordon, which displays interesting antifungal, antifertility, and cytotoxic activity against multidrug resistant cell lines. Our synthesis utilizes a highly efficient metal-catalyzed [5 + 2] vinylcyclopropane-alkyne intramolecular cycloaddition to construct the polyhydroazulene core of the natural product. Elaboration to the tricyclic scaffold of the pseudolaric acids was completed with an intramolecular alkoxycarbonyl radical cyclization to form the quaternary center and a highly diastereoselective cerium acetylide addition to a methyl ketone for introduction of the acid side chain.
Figures



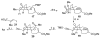
References
-
- Yao JX, Lin XY. Acta Chim Sin (Engl Ed) 1982;40:385.
- Zhou BN, Ying BP, Song GQ, Chen ZX, Han J, Yan YF. Planta Med. 1983;47:35. - PubMed
-
- Wong VKW, Chiu P, Chung SSM, Chow LMC, Zhao YZ, Yang BB, Ko BCB. Clin Cancer Res. 2005;11:6002. - PubMed
-
- Pan BC, Chang HY, Cai GL, Guo YS. Pure Appl Chem. 1989;61:389.
- Higuchi RI. PhD Thesis. Stanford University; 1995.
- Chiu P, Chen B, Cheng KF. Tetrahedron Lett. 1998;39:9229.
- Hu YH, Ou LG, Wang XL, Bai DL. Chin Chem Lett. 1999;10:281.
- Chiu P, Chen B, Cheng KF. Org Lett. 2001;3:1721. - PubMed
- Jiang XT, Ou LG, Han DM, Zhai YF, Bai DL. Chin Chem Lett. 2001;12:113.
- Wu BG, Karle JM, Watkins EB, Avery MA. Tetrahedron Lett. 2002;43:4095.
- Geng Z, Chen B, Chiu P. Angew Chem, Int Ed. 2006;45:6197. - PubMed
-
- Wender PA, Takahashi H, Witulski B. J Am Chem Soc. 1995;117:4720.
- Wender PA, Williams TJ. Angew Chem, Int Ed. 2002;41:4550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

